Global Viral Inactivation Market: Snapshot
A popular strategy with savvy players in the global market for viral inactivation trying to bolster their positions is the thrust on acquisitions. Besides, they are also investing heavily in research and development to come up with new products in response to various requirements for treating diseases. A dominant players in the market, namely Merck & Co., Inc., is seen to have a stronghold across nations worldwide.
At the forefront of driving growth in the global market for viral inactivation is the rising approvals of new drugs, rules pertaining to viral safety of biologics, and the proliferation of the biologics and biosimilars industry. Besides, the growing instances of various infectious diseases and growing application of biosimilars therapeutic treatment for a range of diseases are also positively impacting the market.
Proving counterproductive to the market, on the downside, is the high costs involved in developing and manufacturing biologics and biosimilars products and also the high price of equipment for viral inactivation. However, new types of viruses needing vaccinations to be developed against them spells new opportunities for the market.
A report by Transparency Market Research predicts the global viral inactivation market to expand at a healthy 7.4% CAGR from 2017 to 2025 to become worth US$5.736 bn by 2025 from US$3.05 bn in 2016.
Effectiveness of Chemical Method Drives it to Fore Vis-à-vis Market Share
Depending upon the type of method, the global viral inactivation market can be divided segmented into chemical, radiation, and others such as pasteurization and dry and moist heat treatment. Of them, the chemical method leads the market with maximum share as it is a robust and effective method of inactivation of different types of viruses. It is also cost-effective, safe, and brings about a high degree of purity of sample. The chemical method is further sub-segmented into solvent detergent method, use of alkylating agent method, and pH concentration method. The solvent detergent method, among them, is projected to account for largest market share by the end of 2025.
In terms of growth rate, however, the radiation method for viral inactivation is expected to outpace all others on account of the rising adoption of the method in blood centers in developed nations. The UV radiation method has been tested and developed for viral inactivation of blood and blood components at blood centers and at commercial manufacturing facilities.
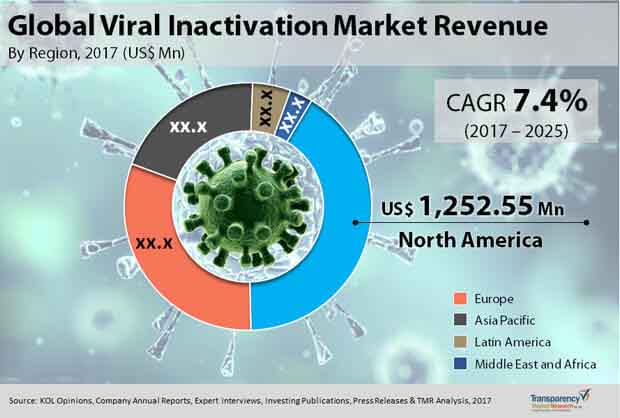
Robust Biopharmaceutical Industry Makes North America Dominant Region
From a geographical standpoint, North America currently holds a sway over the global market on account of a robust biopharmaceutical industry, swift uptake of newer viral inactivation methodologies, rising demand for blood and blood components for transfusion therapy, and strict regulatory guidelines for the viral safety of biologics products. By growing at a CAGR of 7.2% in the forecast period, the market will likely become worth US$2.31 bn by 2025.
However, North America is slated to lose some of its market share to Asia Pacific, which is predicted to expand at maximum pace. The report by TMR projects the viral inactivation market in the region to clock a CAGR of 7.9% from 2017 to 2025. The region’s growth will be primarily powered by countries of South Korea, Japan, and Australia which have strict rules in place for purity of biologics and biosimilars products and also by China, India, and Malaysia having a booming biopharmaceutical industry.
Some of the dominant players in the global market for viral inactivation are Shandong Weigao Group Medical Polymer Company Limited, Sartorius AG, Cerus Corporation, Macopharma SA, and Terumo BCT, Inc.
Global Viral Inactivation Market to Grow with Advancements in Research Related to COVID-19
As the name suggests, viral inactivation is an important process for ensuring that the patients are not exposed to secondary infections or diseases. The occurrence or birth of a virus inside the human body can magnify into a major problem if it is not heeded to at the right time. Therefore, the medical industry is making ardent efforts to ensure that viruses contracted by the human and animal bodies are controlled with adequate procedures. In addition to this, the importance of treating viral infections with palpable ease has also created new opportunities for growth and advancement across the global viral inactivation market. Over the course of the next decade, the use of viral infections and therapies shall increase by leaps and bounds.
Chapter 1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
Chapter 2. Assumptions and Research Methodology
Chapter 3. Executive Summary: Global Viral Inactivation market
Chapter 4. Market Overview
4.1. Introduction
4.1.1. Viral Inactivation Method Introduction
4.1.2. Industry Evolution / Developments
Chapter 5. Market Dynamics
5.1. Drivers
5.2. Restraints
5.3. Opportunities
5.4. Trends
5.5. Viral Inactivation market Analysis and Forecast, 2017 – 2025
5.5.1. Market Revenue Projections (US$ Mn), 2017-2025
5.6. Porter’s five forces analysis
5.7. Market Outlook
Chapter 6. Global Viral Inactivation market Analysis and Forecasts, by Method
6.1. Key Findings
6.2. Introduction
6.3. Market size (US$ Mn) Forecast, by Method, 2017-2025
6.3.1. Chemical method
6.3.1.1. Solvent & Detergents
6.3.1.2. Alkylating Agents
6.3.1.3. pH Concentration
6.3.2. Radiation Method
6.3.3. Other Methods
6.4. Market Attractiveness Analysis, by Method
6.5. Key Trends
Chapter 7. Global Viral Inactivation market Analysis and Forecasts, by Application
7.1. Key Findings
7.2. Introduction
7.3. Market size (US$ Mn) Forecast, by Application, 2017-2025
7.3.1. Blood and Blood Products
7.3.2. Vaccines
7.3.3. Cell and Tissue Culture
7.4. Market Attractiveness, by Application
Chapter 8. Global Viral Inactivation market Analysis and Forecasts, by End-user
8.1. Key Findings
8.2. Introduction
8.3. Market size (US$ Mn) Forecast by End-user, 2017-2025
8.3.1. Pharmaceutical and Biotechnology Companies
8.3.2. Blood banks and Hospitals
8.3.3. Contract Research Organizations
8.3.4. Others
8.4. Market Attractiveness by End-user
Chapter 9. Global Viral Inactivation market Analysis and Forecasts, by Region
9.1. Key Findings
9.2. Global Market Scenario
9.3. Market Value (US$ Mn) Forecast, by Region, 2017-2025
9.3.1. North America
9.3.2. Europe
9.3.3. Asia Pacific
9.3.4. Latin America
9.3.5. Middle East & Africa
9.4. Market Attractiveness, by Region
Chapter 10. North America Viral Inactivation market Analysis and Forecast
10.1. Key Findings
10.2. Market Overview
10.3. Market Value (US$ Mn) Forecast, by Country, 2017-2025
10.3.1. U.S.
10.3.2. Canada
10.4. Market size (US$ Mn) Forecast, by Method, 2017-2025
10.4.1. Chemical method
10.4.1.1. Solvent & Detergents
10.4.1.2. Alkylating Agents
10.4.1.3. pH Concentration
10.4.2. Radiation Method
10.4.3. Other Methods
10.5. Market size (US$ Mn) Forecast, by Application, 2017-2025
10.5.1. Blood and Blood Products
10.5.2. Vaccines
10.5.3. Cell and Tissue Culture
10.6. Market size (US$ Mn) Forecast by End-user, 2017-2025
10.6.1. Pharmaceutical and Biotechnology Companies
10.6.2. Blood banks and Hospitals
10.6.3. Contract Research Organizations
10.6.4. Others
10.7. Market Attractiveness Analysis
10.7.1. By Country
10.7.2. By Method
10.7.3. By Application
10.7.4. By End-user
10.8. Key Trends
Chapter 11. Europe Viral Inactivation market Analysis and Forecast
11.1. Key Findings
11.2. Market Overview
11.3. Market size (US$ Mn) Forecast By Country, 2017-2025
11.3.1. Germany
11.3.2. U.K.
11.3.3. France
11.3.4. Spain
11.3.5. Italy
11.3.6. Rest of Europe
11.4. Market size (US$ Mn) Forecast, by Method, 2017-2025
11.4.1. Chemical method
11.4.1.1. Solvent & Detergents
11.4.1.2. Alkylating Agents
11.4.1.3. pH Concentration
11.4.2. Radiation Method
11.4.3. Other Methods
11.5. Market size (US$ Mn) Forecast, by Application, 2017-2025
11.5.1. Blood and Blood Products
11.5.2. Vaccines
11.5.3. Cell and Tissue Culture
11.6. Market size (US$ Mn) Forecast by End-user, 2017-2025
11.6.1. Pharmaceutical and Biotechnology Companies
11.6.2. Blood banks and Hospitals
11.6.3. Contract Research Organizations
11.6.4. Others
11.7. Market Attractiveness Analysis
11.7.1. By Country
11.7.2. By Method
11.7.3. By Application
11.7.4. By End-user
11.8. Key Trends
Chapter 12. Asia Pacific Viral Inactivation market Analysis and Forecast
12.1. Key Findings / Developments
12.2. Market Overview
12.3. Market size (US$ Mn) Forecast, by Country, 2017-2025
12.3.1. China
12.3.2. India
12.3.3. Japan
12.3.4. Australia & New Zealand
12.3.5. Rest of Asia Pacific
12.4. Market size (US$ Mn) Forecast, by Method, 2017-2025
12.4.1. Chemical method
12.4.1.1. Solvent & Detergents
12.4.1.2. Alkylating Agents
12.4.1.3. pH Concentration
12.4.2. Radiation Method
12.4.3. Other Methods
12.5. Market size (US$ Mn) Forecast, by Application, 2017-2025
12.5.1. Blood and Blood Products
12.5.2. Vaccines
12.5.3. Cell and Tissue Culture
12.6. Market size (US$ Mn) Forecast by End-user, 2017-2025
12.6.1. Pharmaceutical and Biotechnology Companies
12.6.2. Blood banks and Hospitals
12.6.3. Contract Research Organizations
12.6.4. Others
12.7. Market Attractiveness Analysis
12.7.1. By Country
12.7.2. By Method
12.7.3. By Application
12.7.4. By End-user
12.8. Key Trends
Chapter 13. Latin America Viral Inactivation market Analysis and Forecast
13.1. Key Findings / Developments
13.2. Market Overview
13.3. Market Size (US$ Mn) Forecast, by Country, 2017-2025
13.3.1. Brazil
13.3.2. Mexico
13.3.3. Rest of Latin America
13.4. Market size (US$ Mn) Forecast, by Method, 2017-2025
13.4.1. Chemical method
13.4.1.1. Solvent & Detergents
13.4.1.2. Alkylating Agents
13.4.1.3. pH Concentration
13.4.2. Radiation Method
13.4.3. Other Methods
13.5. Market size (US$ Mn) Forecast, by Application, 2017-2025
13.5.1. Blood and Blood Products
13.5.2. Vaccines
13.5.3. Cell and Tissue Culture
13.6. Market size (US$ Mn) Forecast by End-user, 2017-2025
13.6.1. Pharmaceutical and Biotechnology Companies
13.6.2. Blood banks and Hospitals
13.6.3. Contract Research Organizations
13.6.4. Others
13.7. Market Attractiveness Analysis
13.7.1. By Country
13.7.2. By Method
13.7.3. By Application
13.7.4. By End-user
13.8. Key Trends
Chapter 14. Middle East and Africa Viral Inactivation market Analysis and Forecast
14.1. Key Findings / Developments
14.2. Market Overview
14.3. Market size (US$ Mn) Forecast, by Country, 2017-2025
14.3.1. GCC Countries
14.3.2. South Africa
14.3.3. Rest of Middle East and Africa
14.4. Market size (US$ Mn) Forecast, by Method, 2017-2025
14.4.1. Chemical method
14.4.1.1. Solvent & Detergents
14.4.1.2. Alkylating Agents
14.4.1.3. pH Concentration
14.4.2. Radiation Method
14.4.3. Other Methods
14.5. Market size (US$ Mn) Forecast, by Application, 2017-2025
14.5.1. Blood and Blood Products
14.5.2. Vaccines
14.5.3. Cell and Tissue Culture
14.6. Market size (US$ Mn) Forecast by End-user, 2017-2025
14.6.1. Pharmaceutical and Biotechnology Companies
14.6.2. Blood banks and Hospitals
14.6.3. Contract Research Organizations
14.6.4. Others
14.7. Market Attractiveness Analysis
14.7.1. By Country
14.7.2. By Method
14.7.3. By Application
14.7.4. By End-user
14.8. Key Trends
Chapter 15. Competition Landscape
15.1. Market Player – Competition Matrix (By Tier and Size of companies)
15.2. Company Profiles (Details – Overview, Financials, Recent Developments, Strategy)
15.2.1. Parker Hannifin Corporation
15.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.1.2. Product Portfolio
15.2.1.3. SWOT Analysis
15.2.1.4. Strategic Overview
15.2.2. Merck & Co., Inc.
15.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.2.2. Product Portfolio
15.2.2.3. SWOT Analysis
15.2.2.4. Strategic Overview
15.2.3. Shandong Weigao Group Medical Polymer Company Limited
15.2.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.3.2. Product Portfolio
15.2.3.3. SWOT Analysis
15.2.3.4. Financial Overview
15.2.3.5. Strategic Overview
15.2.4. Sartorius AG
15.2.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.4.2. Product Portfolio
15.2.4.3. SWOT Analysis
15.2.4.4. Financial Overview
15.2.4.5. Strategic Overview
15.2.5. Cerus Corporation
15.2.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.5.2. Product Portfolio
15.2.5.3. SWOT Analysis
15.2.5.4. Financial Overview
15.2.5.5. Strategic Overview
15.2.6. Macopharma SA
15.2.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.6.2. Product Portfolio
15.2.6.3. SWOT Analysis
15.2.6.4. Financial Overview
15.2.6.5. Strategic Overview
15.2.7. Terumo BCT, Inc.
15.2.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.7.2. Product Portfolio
15.2.7.3. SWOT Analysis
15.2.7.4. Financial Overview
15.2.7.5. Strategic Overview
15.2.8. Thermo Fisher Scientific Inc.
15.2.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.8.2. Product Portfolio
15.2.8.3. SWOT Analysis
15.2.8.4. Financial Overview
15.2.8.5. Strategic Overview
15.2.9. V.I.P.S. SA
15.2.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.9.2. Product Portfolio
15.2.9.3. SWOT Analysis
15.2.9.4. Financial Overview
15.2.9.5. Strategic Overview
List of Tables
Table 01: Global Viral Inactivation Market Size (US$ Mn) Forecast, by Method, 2015–2025
Table 02: Global Market Size (US$ Mn) Forecast, Chemical Method, 2015–2025
Table 03: Global Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 04: Global Market Size (US$ Mn) Forecast, by End-user, 2015–2025
Table 05: Global Market Size (US$ Mn) Forecast, by Region, 2015–2025
Table 06: North America Viral Inactivation Market Size (US$ Mn) Forecast, by Country/ Sub-region, 2015–2025
Table 07: North America Market Size (US$ Mn) Forecast, by Method, 2015–2025
Table 08: North America Market Size (US$ Mn) Forecast, by Chemical method, 2015–2025
Table 09: North America Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 10: North America Market Size (US$ Mn) Forecast, by End-user, 2015–2025
Table 11: Europe Viral Inactivation Market Value (US$ Mn) Forecast, by Country / Sub-region, 2015–2025
Table 12: Europe Market Value (US$ Mn) Forecast, by Method, 2015–2025
Table 13: Europe Market Size (US$ Mn) Forecast, by Chemical Method, 2015–2025
Table 14: Europe Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 15: Europe Market Size (US$ Mn) Forecast, by End-user, 2015–2025
Table 16: Asia Pacific Viral Inactivation Market Value (US$ Mn) Forecast, by Country/ Sub-region, 2015–2025
Table 17: Asia Pacific Market Value (US$ Mn) Forecast, by Method, 2015–2025
Table 18: Asia Pacific Market Size (US$ Mn) Forecast, by Chemical Method, 2015–2025
Table 19: Asia Pacific Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 20: Asia Pacific Market Size (US$ Mn) Forecast, by End-user, 2015–2025
Table 21: Latin America Viral Inactivation Market Size (US$ Mn) Forecast, by Country/ Sub-region, 2015–2025
Table 22: Latin America Market Size (US$ Mn) Forecast, by Method, 2015–2025
Table 23: Latin America Market Size (US$ Mn) Forecast, by Chemical Method, 2015–2025
Table 24: Latin America Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 25: Latin America Market Size (US$ Mn) Forecast, by End-user, 2015–2025
Table 26: Middle East & Africa Viral Inactivation Market Size (US$ Mn) Forecast, by Country/ Sub-region, 2015–2025
Table 27: Middle East & Africa Market Size (US$ Mn) Forecast, by Method, 2015–2025
Table 28: Middle East & Africa Market Size (US$ Mn) Forecast, by Chemical Method, 2015–2025
Table 29: Middle East & Africa Market Size (US$ Mn) Forecast, by Application, 2015–2025
Table 30: Middle East & Africa Market Size (US$ Mn) Forecast, by End-user, 2015–2025
List of Figures
Figure 01: Global Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2025
Figure 02: Global Market Value Share, by Method (2016)
Figure 03: Global Market Value Share, by Application (2016)
Figure 04: Global Market Value Share, by End-user (2016)
Figure 05: Global Market Value Share, by Region (2016)
Figure 06: Global Market Value Share Analysis, by Method, 2016 and 2025
Figure 07: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical Method, 2016–2025
Figure 08: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation Method, 2016–2025
Figure 09: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Other Methods, 2016–2025
Figure 10: Global Market Attractiveness Analysis, by Method, 2017–2025
Figure 11: Global Market Value Share Analysis, by Application, 2016 and 2025
Figure 12: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood and Blood Products, 2017–2025
Figure 13: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Vaccines, 2017–2025
Figure 14: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Cell & Tissue Culture, 2017–2025
Figure 15: Global Market Attractiveness Analysis, by Application, 2017–2025
Figure 16: Global Market Value Share Analysis, by End-user, 2016 and 2025
Figure 17: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pharmaceutical and Biotechnology companies, 2016–2025
Figure 18: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood Banks and Hospitals, 2016–2025
Figure 19: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Contract Research Organizations, 2016–2025
Figure 20: Global Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2016–2025
Figure 21: Global Market Attractiveness
Figure 22: Global Market Value Share Analysis, by Region, 2016 and 2025
Figure 23: Global Market Attractiveness Analysis, by Region, 2017–2025
Figure 24: North America Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2016–2025
Figure 25: North America Market Attractiveness Analysis, by Country/Sub-region, 2017–2025
Figure 26: North America Market Value Share Analysis, by Country/ Sub-region, 2016 and 2025
Figure 27: North America Market Value Share Analysis, by Method, 2016 and 2025
Figure 28: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical method, 2016–2025
Figure 29: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation method, 2016–2025
Figure 30: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Other Methods, 2016–2025
Figure 31: North America Market Value Share Analysis, by Application, 2016 and 2025
Figure 32: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood and Blood Products, 2016–2025
Figure 33: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Vaccines, 2016–2025
Figure 34: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Cell & Tissue Culture, 2016–2025
Figure 35: North America Market Value Share Analysis, by End-user, 2016 and 2025
Figure 36: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pharmaceutical and Biotechnology Companies, 2016–2025
Figure 37: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood Banks and Hospitals, 2016–2025
Figure 38: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Contract Research Organizations, 2016–2025
Figure 39: North America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2016–2025
Figure 40: North America Market Attractiveness Analysis, by Method, 2017–2025
Figure 41: North America Market Attractiveness Analysis, by Application, 2017–2025
Figure 42: North America Market Attractiveness Analysis, by End-user, 2017-2025
Figure 43: Europe Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2016–2025
Figure 44: Europe Market Attractiveness Analysis, by Country / Sub-region, 2017–2025
Figure 45: Europe Market Value Share Analysis, by Country / Sub-region, 2016 and 2025
Figure 46: Europe Market Value Share Analysis, by Method, 2016 and 2025
Figure 47: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical, 2016–2025
Figure 48: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation Method, 2016–2025
Figure 49: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Other Methods, 2016–2025
Figure 50: Europe Market Value Share Analysis (US$ Mn), by Application, 2016 and 2025
Figure 51: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood & Blood Products, 2016–2025
Figure 52: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Vaccines, 2016–2025
Figure 53: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Cell & Tissue Culture, 2016–2025
Figure 54: Europe Market Value share Analysis, by End-user, 2016 and 2025
Figure 55: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pharmaceutical & Biotechnology Companies, 2016–2025
Figure 56: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood Banks & Hospitals, 2016–2025
Figure 57: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Contract Research Organization, 2016–2025
Figure 58: Europe Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2016–2025
Figure 59: Europe Market Attractiveness Analysis, by Method, 2017–2025
Figure 60: Europe Market Attractiveness Analysis, by Application, 2017–2025
Figure 61: Europe Market Attractiveness Analysis, by End-user, 2017–2025
Figure 62: Asia Pacific Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2016–2025
Figure 63: Asia Pacific Market Attractiveness Analysis, by Country/Sub-region, 2017–2025
Figure 64: Asia Pacific Market Value Share Analysis, by Country/Sub-region, 2016 and 2025
Figure 65: Asia Pacific Market Value Share Analysis, by Method, 2016 and 2025
Figure 66: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical Method, 2016–2025
Figure 67: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation Method, 2016–2025
Figure 68: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Other Methods, 2016–2025
Figure 69: Asia Pacific Market Value Share Analysis, by Application, 2016 and 2025
Figure 70: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood & Blood Products, 2016–2025
Figure 71: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Vaccines, 2016–2025
Figure 72: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Cell & Tissue Culture, 2016–2025
Figure 73: Asia Pacific Market Value share Analysis, by End-user, 2016 and 2025
Figure 74: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pharmaceutical & Biotechnology Companies, 2016–2025
Figure 75: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood Banks & Hospitals, 2016–2025
Figure 76: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Contract Research Organizations, 2016–2025
Figure 77: Asia Pacific Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2016–2025
Figure 78: Asia PacificMarket Attractiveness Analysis, by Method, 2017–2025
Figure 79: Asia Pacific Market Attractiveness Analysis, by Application, 2017–2025
Figure 80: Asia Pacific Market Attractiveness Analysis, by End-user, 2017–2025
Figure 81: Latin America Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2016–2025
Figure 82: Latin America Market Attractiveness Analysis, by Country/Sub-region, 2017-2025
Figure 83: Latin America Market Value Share Analysis, by Country/Sub-region, 2016 and 2025
Figure 84: Latin America Market Value Share Analysis, by Method, 2016 and 2025
Figure 85: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical Method, 2016–2025
Figure 86: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation method, 2016–2025
Figure 87: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by other methods 2016–2025
Figure 88: Latin America Market Value Share Analysis, by Application, 2016 and 2025
Figure 89: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood and Blood Products 2016–2025
Figure 90: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Vaccines, 2016–2025
Figure 91: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Cell and Tissue Culture, 2016–2025
Figure 92: Latin America Market Value Share Analysis, by End-user, 2016 and 2025
Figure 93: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Pharmaceutical and Biotechnology companies, 2016–2025
Figure 94: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Blood Banks and Hospitals, 2016–2025
Figure 95: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Contract Research Organizations 2016–2025
Figure 96: Latin America Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Others, 2016–2025
Figure 97: Latin America Market Attractiveness Analysis, by Method, 2017–2025
Figure 98: Latin America arket Attractiveness Analysis, by Application, 2017–2025
Figure 99: Latin America Market Attractiveness Analysis, by End-user, 2017-2025
Figure 100: Middle East & Africa Viral Inactivation Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2016–2025
Figure 101: Middle East & Africa Market Attractiveness Analysis, by Country/Sub-region, 2017-2025
Figure 102: Middle East & Africa Market Value Share Analysis, by Country/Sub-region, 2016 and 2025
Figure 103: Middle East & Africa Market Value Share Analysis, by Method, 2016 and 2025
Figure 104: Middle East & Africa Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Chemical Method, 2016–2025
Figure 105: Middle East & Africa Market Revenue (US$ Mn) and Y-o-Y Growth (%), by Radiation method, 2016–2025
Figure 106: Middle East & Africa Market Revenue (US$ Mn) and Y-o-Y Growth (%), by other methods, 2016–2025
Figure 107: Middle East & Africa Market Value Share Analysis, by Application, 2016 and 2025
Figure 108: Middle East & Africa Blood and Blood Products Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 109: Middle East & Africa Vaccines Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 110: Middle East & Africa Cell Culture Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 111: Middle East & Africa Market Value Share Analysis, by End-user, 2016 and 2025
Figure 112: Middle East & Africa Pharmaceutical and Biotechnology companies Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 113: Middle East & Africa Blood Banks and Hospitals Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 114: Middle East & Africa Contract Research Organizations Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 115: Middle East & Africa Others Market Revenue (US$ Mn) and Y-o-Y Growth (%), 2016–2025
Figure 116: Middle East & Africa Market Attractiveness Analysis, by Method, 2017–2025
Figure 117: Middle East & Africa Market Attractiveness Analysis, by Application, 2017–2025
Figure 118: Middle East & Africa Market Attractiveness Analysis, by End-user, 2017–2025