Analyst Viewpoint
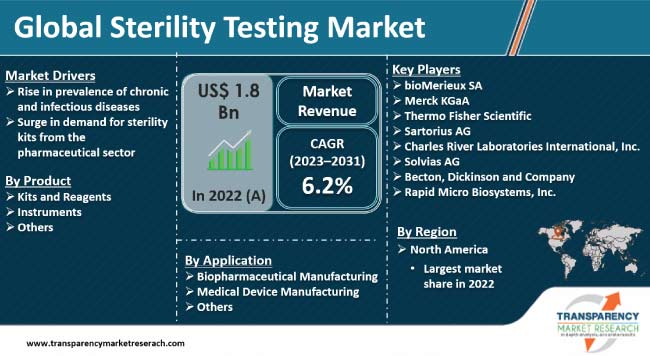
Rise in prevalence of chronic and infectious diseases among people and increase in demand for sterility kits from the pharmaceutical sector are augmenting the sterility testing market size. Sterility kits can precisely detect the presence of microorganisms on various medical equipment to avoid the spread of infectious diseases. Growth in adoption of rapid sterility testing to provide quick treatments to patients is augmenting the sterility testing industry value.
Leading companies in the market are forming partnerships with companies operating in the pharmaceutical sector and undertaking acquisitions. This strategy allows companies to develop sterility kits according to their requirements and ensure high performance and result accuracy. Furthermore, manufacturers are investing heavily in research and development activities to adopt advanced technologies and launch new testing kits.
Sterility testing refers to a set of activities performed to confirm that products are free from microorganisms. The sterility testing method involves passing a quantity of drug product through filters designed to retain microorganisms. It is followed by a rinse to ensure that no drug product remains on the filter, which can inhibit the growth of microorganisms. These testing methods are extensively employed in the pharmaceutical sector to limit the spread of viruses.
Membrane filtration and direct inoculation are two major types of sterility testing. Membrane filtration sterility testing is the regulatory method of choice for filterable pharmaceutical products, whereas in direct inoculation, a small volume of sample is removed aseptically from the sample unit and inoculated directly into a suitable volume of growth medium before incubation. Accurate determination of purity, hygiene maintenance, disinfecting medical devices, and safety of drugs are key advantages of sterility testing.
Poor sanitation, lack of vaccination, or exposure to infected individuals are causing infectious diseases such as flu, tuberculosis, and human immunodeficiency virus. Changes in lifestyles, eating habits, and lack of physical activities are causing chronic diseases among the geriatric population. Sterility testing is a crucial step to prevent the spread of these diseases by ensuring absence of viable microorganisms in drugs, medical equipment, and other medical devices required during treatments. Thus, rise in prevalence of infectious and chronic diseases is augmenting the sterility testing market revenue.
Increase in demand for rapid sterility testing in the pharmaceutical sector is offering lucrative opportunities for market expansion. Rapid sterility testing has shortened incubation times and reduced subjectivity in result analysis. Growth in popularity of rapid drug testing to ensure quick treatments is creating high demand for sterility testing tools.
Sterility kits are widely utilized in the pharmaceutical sector to assess the purity of drugs, diagnosis kits, and ensure sanitization. The World Health Organization (WHO) sterility testing guidelines are applicable across a wide range of biological medicinal products, such as biotechnology products, vaccines, cell & tissue products, and blood products. Hence, increase in demand for sterility testing kits in the pharmaceutical sector to ensure purity of various products is bolstering the sterility testing market growth.
Increase in adoption of sterile product testing in biopharmaceuticals is driving the demand for sterility testing kits. Adenosine Triphosphate (ATP) bioluminescence is one of the common rapid sterility testing methods for pharmaceuticals that provides accurate results in a short period of time. Furthermore, increase in investments in drug development and clinical trials by pharmaceutical companies is increasing the use of sterility testing tools.
As per the latest regional sterility testing market analysis, North America dominated the global landscape in 2022. Early adoption of advanced technologies and establishment of new biopharmaceutical companies in the region are likely to propel the market share in the next few years. Increase in investments in research and development activities in medical technology and life sciences is fueling the sterility testing industry revenue.
Leading companies in the region are introducing new drugs for prevention of infectious diseases. For instance, in March 2022, VBI Vaccines Inc. launched PreHevbrioin in the United States for the prevention of infection caused by all known subtypes of Hepatitis B Virus (HBV) in adults.
Leading companies in the market are implementing advanced business strategies, such as acquisitions and partnerships to increase their presence in global markets. Manufacturers are adopting the latest sterility testing market trends to launch new testing kits with advanced technologies. They are investing in research and development activities to launch new products with high accuracy and provide results in minimum time.
Some of the leading players in the industry include bioMerieux SA, Merck KGaA, Thermo Fisher Scientific, Sartorius AG, Charles River Laboratories International, Inc., Solvias AG, Becton, Dickinson and Company, and Rapid Micro Biosystems, Inc.
These companies are profiled in the sterility testing market report based on various parameters including company overview, business segments, product portfolio, recent developments, business strategies, and financial overview.
| Attribute | Detail |
|---|---|
| Market Size in 2022 | US$ 1.8 Bn |
| Market Forecast (Value) in 2031 | US$ 3.1 Bn |
| Growth Rate (CAGR) | 6.2% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Tons | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces Analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Market Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 1.8 Bn in 2022
It is projected to register a CAGR of 6.2% from 2023 to 2031
Rise in prevalence of chronic and infectious diseases and rise in demand for sterility kits from the pharmaceutical sector
North America was the most lucrative region in 2022
bioMerieux SA, Merck KGaA, Thermo Fisher Scientific, Sartorius AG, Charles River Laboratories International, Inc., Solvias AG, Becton, Dickinson and Company, and Rapid Micro Biosystems, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Sterility Testing Market
4. Market Overview
4.1. Introduction
4.1.1. Product Definition
4.1.2. Industry Evolution/Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Sterility Testing Market Analysis and Forecast, 2017–2031
5. Key Insights
5.1. Pipeline Analysis
5.2. Key Product/Brand Analysis
5.3. Key Mergers & Acquisitions
5.4. COVID-19 Pandemic Impact on Industry
6. Global Sterility Testing Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Product, 2017–2031
6.3.1. Kits and Reagents
6.3.2. Instruments
6.3.3. Others
6.4. Market Attractiveness Analysis, by Product
7. Global Sterility Testing Market Analysis and Forecast, by Application
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Application, 2017–2031
7.3.1. Biopharmaceutical Manufacturing
7.3.2. Medical Device Manufacturing
7.3.3. Others
7.4. Market Attractiveness Analysis, by Application
8. Global Sterility Testing Market Analysis and Forecast, by Test Type
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Test Type, 2017–2031
8.3.1. Traditional Sterility Tests
8.3.1.1. Membrane Filtration
8.3.1.2. Immersion Test
8.3.2. Rapid Sterility Tests
8.3.2.1. Solid Phase Cytometry
8.3.2.2. Flow Cytometry
8.3.2.3. Bioluminescence
8.3.2.4. Nucleic Acid Amplification
8.3.2.5. Immunological Methods
8.3.2.6. Others
8.4. Market Attractiveness Analysis, by Application
9. Global Sterility Testing Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2017–2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Sterility Testing Market Analysis and Forecast
10.1. Introduction
10.2. Key Findings
10.3. Market Value Forecast, by Product, 2017–2031
10.3.1. Kits and Reagents
10.3.2. Instruments
10.3.3. Others
10.4. Market Value Forecast, by Application, 2017–2031
10.4.1. Biopharmaceutical Manufacturing
10.4.2. Medical Device Manufacturing
10.4.3. Others
10.5. Market Value Forecast, by Test Type, 2017–2031
10.5.1. Traditional Sterility Tests
10.5.1.1. Membrane Filtration
10.5.1.2. Immersion Test
10.5.2. Rapid Sterility Tests
10.5.2.1. Solid Phase Cytometry
10.5.2.2. Flow Cytometry
10.5.2.3. Bioluminescence
10.5.2.4. Nucleic Acid Amplification
10.5.2.5. Immunological Methods
10.5.2.6. Others
10.6. Market Value Forecast, by Country, 2017–2031
10.6.1. U.S.
10.6.2. Canada
10.7. Market Attractiveness Analysis
10.7.1. By Product
10.7.2. By Application
10.7.3. By Test Type
10.7.4. By Country
11. Europe Sterility Testing Market Analysis and Forecast
11.1. Introduction
11.2. Key Findings
11.3. Market Value Forecast, by Product, 2017–2031
11.3.1. Kits and Reagents
11.3.2. Instruments
11.3.3. Others
11.4. Market Value Forecast, by Application, 2017–2031
11.4.1. Biopharmaceutical Manufacturing
11.4.2. Medical Device Manufacturing
11.4.3. Others
11.5. Market Value Forecast, by Test Type, 2017–2031
11.5.1. Traditional Sterility Tests
11.5.1.1. Membrane Filtration
11.5.1.2. Immersion Test
11.5.2. Rapid Sterility Tests
11.5.2.1. Solid Phase Cytometry
11.5.2.2. Flow Cytometry
11.5.2.3. Bioluminescence
11.5.2.4. Nucleic Acid Amplification
11.5.2.5. Immunological Methods
11.5.2.6. Others
11.6. Market Value Forecast, by Country/Sub-region, 2017–2031
11.6.1. Germany
11.6.2. U.K.
11.6.3. France
11.6.4. Italy
11.6.5. Spain
11.6.6. Rest of Europe
11.7. Market Attractiveness Analysis
11.7.1. By Product
11.7.2. By Application
11.7.3. By Test Type
11.7.4. By Country/Sub-region
12. Asia Pacific Sterility Testing Market Analysis and Forecast
12.1. Introduction
12.2. Key Findings
12.3. Market Value Forecast, by Product, 2017–2031
12.3.1. Kits and Reagents
12.3.2. Instruments
12.3.3. Others
12.4. Market Value Forecast, by Application, 2017–2031
12.4.1. Biopharmaceutical Manufacturing
12.4.2. Medical Device Manufacturing
12.4.3. Others
12.5. Market Value Forecast, by Test Type, 2017–2031
12.5.1. Traditional Sterility Tests
12.5.1.1. Membrane Filtration
12.5.1.2. Immersion Test
12.5.2. Rapid Sterility Tests
12.5.2.1. Solid Phase Cytometry
12.5.2.2. Flow Cytometry
12.5.2.3. Bioluminescence
12.5.2.4. Nucleic Acid Amplification
12.5.2.5. Immunological Methods
12.5.2.6. Others
12.6. Market Value Forecast, by Country/Sub-region, 2017–2031
12.6.1. China
12.6.2. Japan
12.6.3. India
12.6.4. Australia & New Zealand
12.6.5. Rest of Asia Pacific
12.7. Market Attractiveness Analysis
12.7.1. By Product
12.7.2. By Application
12.7.3. By Test Type
12.7.4. By Country/Sub-region
13. Latin America Sterility Testing Market Analysis and Forecast
13.1. Introduction
13.2. Key Findings
13.3. Market Value Forecast, by Product, 2017–2031
13.3.1. Kits and Reagents
13.3.2. Instruments
13.3.3. Others
13.4. Market Value Forecast, by Application, 2017–2031
13.4.1. Biopharmaceutical Manufacturing
13.4.2. Medical Device Manufacturing
13.4.3. Others
13.5. Market Value Forecast, by Test Type, 2017–2031
13.5.1. Traditional Sterility Tests
13.5.1.1. Membrane Filtration
13.5.1.2. Immersion Test
13.5.2. Rapid Sterility Tests
13.5.2.1. Solid Phase Cytometry
13.5.2.2. Flow Cytometry
13.5.2.3. Bioluminescence
13.5.2.4. Nucleic Acid Amplification
13.5.2.5. Immunological Methods
13.5.2.6. Others
13.6. Market Value Forecast, by Country/Sub-region, 2017–2031
13.6.1. Brazil
13.6.2. Mexico
13.6.3. Rest of Latin America
13.7. Market Attractiveness Analysis
13.7.1. By Product
13.7.2. By Application
13.7.3. By Test Type
13.7.4. By Country/Sub-region
14. Middle East & Africa Sterility Testing Market Analysis and Forecast
14.1. Introduction
14.2. Key Findings
14.3. Market Value Forecast, by Product, 2017–2031
14.3.1. Kits and Reagents
14.3.2. Instruments
14.3.3. Others
14.4. Market Value Forecast, by Application, 2017–2031
14.4.1. Biopharmaceutical Manufacturing
14.4.2. Medical Device Manufacturing
14.4.3. Others
14.5. Market Value Forecast, by Test Type, 2017–2031
14.5.1. Traditional Sterility Tests
14.5.1.1. Membrane Filtration
14.5.1.2. Immersion Test
14.5.2. Rapid Sterility Tests
14.5.2.1. Solid Phase Cytometry
14.5.2.2. Flow Cytometry
14.5.2.3. Bioluminescence
14.5.2.4. Nucleic Acid Amplification
14.5.2.5. Immunological Methods
14.5.2.6. Others
14.6. Market Value Forecast, by Country/Sub-region, 2017–2031
14.6.1. GCC Countries
14.6.2. South Africa
14.6.3. Rest of Middle East & Africa
14.7. Market Attractiveness Analysis
14.7.1. By Product
14.7.2. By Application
14.7.3. By Test Type
14.7.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competitive Matrix (by Tier and Size of Companies)
15.2. Market Share Analysis, by Company (2022)
15.3. Company Profiles
15.3.1. bioMerieux SA
15.3.1.1. Company Overview
15.3.1.2. Product Portfolio
15.3.1.3. SWOT Analysis
15.3.1.4. Financial Overview
15.3.1.5. Strategic Overview
15.3.2. Merck KGaA
15.3.2.1. Company Overview
15.3.2.2. Product Portfolio
15.3.2.3. SWOT Analysis
15.3.2.4. Financial Overview
15.3.2.5. Strategic Overview
15.3.3. Thermo Fisher Scientific
15.3.3.1. Company Overview
15.3.3.2. Product Portfolio
15.3.3.3. SWOT Analysis
15.3.3.4. Financial Overview
15.3.3.5. Strategic Overview
15.3.4. Sartorius AG
15.3.4.1. Company Overview
15.3.4.2. Product Portfolio
15.3.4.3. SWOT Analysis
15.3.4.4. Financial Overview
15.3.4.5. Strategic Overview
15.3.5. Charles River Laboratories International, Inc.
15.3.5.1. Company Overview
15.3.5.2. Product Portfolio
15.3.5.3. SWOT Analysis
15.3.5.4. Financial Overview
15.3.5.5. Strategic Overview
15.3.6. Solvias AG
15.3.6.1. Company Overview
15.3.6.2. Product Portfolio
15.3.6.3. SWOT Analysis
15.3.6.4. Financial Overview
15.3.6.5. Strategic Overview
15.3.7. Becton, Dickinson and Company
15.3.7.1. Company Overview
15.3.7.2. Product Portfolio
15.3.7.3. SWOT Analysis
15.3.7.4. Financial Overview
15.3.7.5. Strategic Overview
15.3.8. Rapid Micro Biosystems, Inc.
15.3.8.1. Company Overview
15.3.8.2. Product Portfolio
15.3.8.3. SWOT Analysis
15.3.8.4. Financial Overview
15.3.8.5. Strategic Overview
List of Tables
Table 01: Global Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 02: Global Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 03: Global Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
Table 04: Global Sterility Testing Market Size (US$ Mn) Forecast, by Region, 2017–2031
Table 05: North America Sterility Testing Market Size (US$ Mn) Forecast, by Country, 2017–2031
Table 06: North America Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 07: North America Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 08: North America Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
Table 09: Europe Sterility Testing Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 10: Europe Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 11: Europe Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 12: Europe Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
Table 13: Asia Pacific Sterility Testing Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 14: Asia Pacific Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 15: Asia Pacific Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 16: Asia Pacific Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
Table 17: Latin America Sterility Testing Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 18: Latin America Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 19: Latin America Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 20: Latin America Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
Table 21: Middle East & Africa Sterility Testing Market Size (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 22: Middle East & Africa Sterility Testing Market Size (US$ Mn) Forecast, by Product, 2017–2031
Table 23: Middle East & Africa Sterility Testing Market Size (US$ Mn) Forecast, by Application, 2017–2031
Table 24: Middle East & Africa Sterility Testing Market Size (US$ Mn) Forecast, by Test Type, 2017–2031
List of Figures
Figure 01: Global Sterility Testing Market Size (US$ Mn) and Distribution (%), by Region, 2022 and 2031
Figure 02: Global Sterility Testing Market Revenue (US$ Mn), by Product, 2022
Figure 03: Global Sterility Testing Market Value Share, by Product, 2022
Figure 04: Global Sterility Testing Market Revenue (US$ Mn), by Application, 2022
Figure 05: Global Sterility Testing Market Value Share, by Application, 2022
Figure 06: Global Sterility Testing Market Revenue (US$ Mn), by Test Type, 2022
Figure 07: Global Sterility Testing Market Value Share, by Test Type, 2022
Figure 08: Global Sterility Testing Market Value Share, by Region, 2022
Figure 09: Global Sterility Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 10: Global Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 11: Global Sterility Testing Market Attractiveness Analysis, by Product, 2023-2031
Figure 12: Global Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 13: Global Sterility Testing Market Attractiveness Analysis, by Application, 2023-2031
Figure 14: Global Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 15: Global Sterility Testing Market Attractiveness Analysis, by Test Type, 2023-2031
Figure 16: Global Sterility Testing Market Value Share Analysis, by Region, 2022 and 2031
Figure 17: Global Sterility Testing Market Attractiveness Analysis, by Region, 2023-2031
Figure 18: North America Sterility Testing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2031
Figure 19: North America Sterility Testing Market Attractiveness Analysis, by Country, 2023–2031
Figure 20: North America Sterility Testing Market Value Share Analysis, by Country, 2022 and 2031
Figure 21: North America Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 22: North America Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 23: North America Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 24: North America Sterility Testing Market Attractiveness Analysis, by Product, 2023–2031
Figure 25: North America Sterility Testing Market Attractiveness Analysis, by Application, 2023–2031
Figure 26:North America Sterility Testing Market Attractiveness Analysis, by Test Type, 2023–2031
Figure 27: Europe Sterility Testing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2031
Figure 28: Europe Sterility Testing Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 29: Europe Sterility Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 30: Europe Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 31: Europe Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 32: Europe Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 33: Europe Sterility Testing Market Attractiveness Analysis, by Product, 2023–2031
Figure 34: Europe Sterility Testing Market Attractiveness Analysis, by Application, 2023–2031
Figure 35: Europe Sterility Testing Market Attractiveness Analysis, by Test Type, 2023–2031
Figure 36: Asia Pacific Sterility Testing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2031
Figure 37: Asia Pacific Sterility Testing Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 38: Asia Pacific Sterility Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 39: Asia Pacific Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 40: Asia Pacific Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 41: Asia Pacific Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 42: Asia Pacific Sterility Testing Market Attractiveness Analysis, by Product, 2023–2031
Figure 43: Asia Pacific Sterility Testing Market Attractiveness Analysis, by Application, 2023–2031
Figure 44: Asia Pacific Sterility Testing Market Attractiveness Analysis, by Test Type, 2023–2031
Figure 45: Latin America Sterility Testing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2031
Figure 46: Latin America Sterility Testing Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 47: Latin America Sterility Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 48: Latin America Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 49: Latin America Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 50: Latin America Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 51: Latin America Sterility Testing Market Attractiveness Analysis, by Product, 2023–2031
Figure 52: Latin America Sterility Testing Market Attractiveness Analysis, by Application, 2023–2031
Figure 53: Latin America Sterility Testing Market Attractiveness Analysis, by Test Type, 2023–2031
Figure 54: Middle East & Africa Sterility Testing Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2017–2031
Figure 55: Middle East & Africa Sterility Testing Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 56: Middle East & Africa Sterility Testing Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 57: Middle East & Africa Sterility Testing Market Value Share Analysis, by Product, 2022 and 2031
Figure 58: Middle East & Africa Sterility Testing Market Value Share Analysis, by Application, 2022 and 2031
Figure 59: Middle East & Africa Sterility Testing Market Value Share Analysis, by Test Type, 2022 and 2031
Figure 60: Middle East & Africa Sterility Testing Market Attractiveness Analysis, by Product, 2023–2031
Figure 61: Middle East & Africa Sterility Testing Market Attractiveness Analysis, by Application, 2023–2031
Figure 62: Middle East & Africa Sterility Testing Market Attractiveness Analysis, by Test Type, 2023–2031