Analysts’ Viewpoint on Market Scenario
Preeclampsia is a pregnancy-specific, multisystem disorder characterized by hypertension (>140/90 mm Hg) accompanied by new onset of proteinuria (>300 mg in 24 hours) after 20 weeks’ gestation. Increase in prevalence of preeclampsia across the globe is estimated to propel the preeclampsia diagnostics market progress in the near future. Preeclampsia has an impact on 2% to 8% of pregnancies worldwide. Increase in awareness about this disorder and its diagnostic procedures in pregnant women is anticipated to boost preeclampsia diagnostics market expansion in the next few years. Companies operating in the market are focusing on research and development activities to develop technologically advanced products to gain revenue benefits.
Preeclampsia is a special complication of pregnancy. A systolic blood pressure of at least 140 mm Hg or a diastolic blood pressure of at least 90 mm Hg during pregnancy, as well as protein in the urine (proteinuria), both of which appear after week 20 of pregnancy, are required for its diagnosis. If there is only newly developed hypertension, the condition is known as pregnancy-induced hypertension (without proteinuria or other signs of organ affection).
Before the 20th week of pregnancy, some women experience high blood pressure, which is known as chronic hypertension. Preeclampsia and chronic hypertension are the common hypertensive issues of pregnancy. Preeclampsia typically develops at the end of pregnancy with little to no symptoms.
A more severe form of the condition, which can include complications such as eclampsia (fits), cerebral haemorrhage (brain bleeding), pulmonary edoema (fluid collections in the lungs), kidney failure, liver damage, and serious issues with the blood clotting system, develops in about 1 in 100 women with preeclampsia.
Preeclampsia is a common condition in pregnant women that is becoming more prevalent globally. Preeclampsia diagnostic tests are required due to a rise in blood pressure during pregnancy brought on by changing lifestyles, poor diet, inactivity, alcohol use, and mental stress. Incidence of preeclampsia has grown in the U.S. due to risk factors including advanced maternal age, obesity, and diabetes.
According to the Centers for Disease Control and Prevention, the number of incidences of preeclampsia among childbirth hospitalizations grew from roughly 13% in 2017 to 16% in 2019, affecting at least 1 in 7 deliveries during this time.
Preeclampsia was a documented condition in about one-third of the individuals who died during hospital birth. Preeclampsia was more common (31% more likely) in hospital deliveries for women over 45. This condition was especially prevalent in rural counties (16%) and low-income ZIP codes (16%), as well as in women who gave birth in hospitals in the South (16%) or the Midwest (15%). Therefore, rapid growth in patients with preeclampsia is contributing to the preeclampsia diagnostics market progress during the forecast period.
The preeclampsia diagnostics industry is primarily driven by heavy investments in preeclampsia research and development by numerous foundations and organizations during the forecast period. For instance, the Preeclampsia Foundation and its Canada-based affiliate, Preeclampsia Foundation Canada, would each give one medical research Vision Grant, up to US$ 20,000, on February 2, 2022, to study preeclampsia and related hypertension disorders of pregnancy.
The Preeclampsia Foundation revealed on January 27, 2022, that MoMMA's Voices, a national alliance of patient advocacy groups and people with "lived experience “(or those who represent them), had received new financing from Merck for Mothers. Merck for Mothers is a global program by Merck that aims to prevent maternal deaths. Hence, increase in funding for preeclampsia research & diagnosis by governments and other organizations is anticipated to propel the preeclampsia diagnostics market development during the forecast period.
The blood test segment dominated the global market of preeclampsia diagnostics with more than 55% share in 2021. Preeclampsia can be accurately predicted by biochemical testing. The tests are extremely helpful in predicting preeclampsia both in the first trimester and in the subsequent trimesters, particularly in cases of an early beginning of the disease.
Based on product & services, the preeclampsia diagnostics business is divided into instruments, reagents & consumables, and services. The services segment is projected to dominate the global market of preeclampsia diagnostics, and is anticipated to grow at a high CAGR during the forecast period. Services include expenses occurring in the diagnostic laboratory such as rent/EMI on property, staff salary, pathologist’s salary, sample collection, report printing, etc.
The hospital segment held maximum share of the preeclampsia diagnostics industry in 2021, and is anticipated to grow at a high CAGR from 2022 to 2031. In case of severe preeclampsia, patients are more likely to opt for hospitals for diagnosis and treatment. Patients admitted to hospitals are monitored regularly by taking urine and blood samples. This is estimated to fuel the hospitals segment during the forecast period.
North America accounted for dominating share of 35% of the global market in 2021. The market in the region is projected to grow at a CAGR of over 3.6% during the forecast period. Rise in awareness of preeclampsia diagnosis in pregnant women is contributing to the market growth in the region. Several organizations and major players are increasingly focusing on research and development activities to develop advanced products to detect preeclampsia. This is expected to create lucrative opportunities for market players in the North America region during the forecast period.
Asia Pacific was the fastest-growing market in 2021. The market in the region is expected to grow at a CAGR of 4.4% during the forecast period. This can be ascribed to the rise in prevalence of preeclampsia; increase in population of India and China; availability and awareness of preeclampsia tests; and improvement in healthcare infrastructure in the Asia Pacific region. Additionally, increase in awareness about preeclampsia and its treatment is expected to drive the market in the region during the forecast period.
The global market for preeclampsia diagnostics is consolidated, with a few leading players that control majority of the share. Most companies are investing significantly in research & development. Expansion of product portfolios and mergers & acquisitions are notable strategies adopted by key players. Key players operating in the market include Abbott Laboratories, ACON Laboratories, Inc., ARKRAY, Inc., Bio-Rad Laboratories, Inc., Cardinal Health, Danaher Corporation (Beckman Coulter, Inc.), Diabetomics, Inc., DIRUI Industrial Co., Ltd., F. Hoffmann-La Roche Ltd., Metabolomic Diagnostics Ltd., Sera Prognostics, and Siemens Healthineers AG.
Key players have been profiled in the market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
|
Attribute |
Detail |
|
Market Size Value in 2021 |
US$ 1.5 Bn |
|
Market Forecast Value in 2031 |
US$ 2.3 Bn |
|
Growth Rate |
3.8% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2020 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes cross segment analysis as well as at regional level. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
Market share analysis by company (2021) Company profiles section includes overview, product portfolio, sales footprint, key subsidiaries or distributors, strategy & recent developments, and key financials. |
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
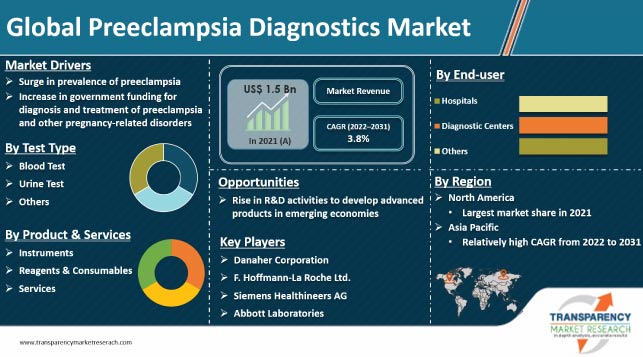
The market was valued at US$ 1.5 Bn in 2021
It is expected to grow at a CAGR of 3.8% from 2022 to 2031
The global market would be worth US$ 2.3 Bn in 2031
Surge in prevalence of preeclampsia and increase in government funding for diagnosis and treatment of preeclampsia and other pregnancy-related disorders
The blood test segment held largest share of 55% in 2021
North America accounted major share of the global market in 2021
Abbott Laboratories, ACON Laboratories, Inc., ARKRAY, Inc., Bio-Rad Laboratories, Inc., Cardinal Health, Danaher Corporation (Beckman Coulter, Inc.), Diabetomics, Inc., DIRUI Industrial Co., Ltd., F. Hoffmann-La Roche Ltd., Metabolomic Diagnostics Ltd., Sera Prognostics, and Siemens Healthineers AG
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Preeclampsia Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Preeclampsia Diagnostics Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Technological Advancements
5.2. Disease Prevalence & Incidence Rate globally with key countries
5.3. Regulatory Scenario by Region/globally
5.4. Key Industry Events (Mergers, Acquisitions, Partnerships, Collaborations, etc.)
5.5. COVID-19 Pandemic Impact on Industry
6. Global Preeclampsia Diagnostics Market Analysis and Forecast, by Test Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Test Type, 2017–2031
6.3.1. Blood Test
6.3.2. Urine Test
6.3.3. Others
6.4. Market Attractiveness By Test Type
7. Global Preeclampsia Diagnostics Market Analysis and Forecast, by Product & Services
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Product & Services, 2017–2031
7.3.1. Instruments
7.3.2. Reagents & Consumables
7.3.3. Services
7.4. Market Attractiveness By Product & Services
8. Global Preeclampsia Diagnostics Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by End-user, 2017–2031
8.3.1. Hospitals
8.3.2. Diagnostic Centers
8.3.3. Others
8.4. Market Attractiveness By End-user
9. Global Preeclampsia Diagnostics Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness By Country/Region
10. North America Preeclampsia Diagnostics Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Test Type, 2017–2031
10.2.1. Blood Test
10.2.2. Urine Test
10.2.3. Others
10.3. Market Value Forecast, by Product & Services, 2017–2031
10.3.1. Instruments
10.3.2. Reagents & Consumables
10.3.3. Services
10.4. Market Value Forecast, by End-user, 2017–2031
10.4.1. Hospitals
10.4.2. Diagnostic Centers
10.4.3. Others
10.5. Market Value Forecast, by Country, 2017–2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Test Type
10.6.2. By Product & Services
10.6.3. By End-user
10.6.4. By Country
11. Europe Preeclampsia Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Test Type, 2017–2031
11.2.1. Blood Test
11.2.2. Urine Test
11.2.3. Others
11.3. Market Value Forecast, by Product & Services, 2017–2031
11.3.1. Instruments
11.3.2. Reagents & Consumables
11.3.3. Services
11.4. Market Value Forecast, by End-user, 2017–2031
11.4.1. Hospitals
11.4.2. Diagnostic Centers
11.4.3. Others
11.5. Market Value Forecast, by Country/Sub-Region, 2017–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Test Type
11.6.2. By Product & Services
11.6.3. By End-user
11.6.4. By Country/Sub-Region
12. Asia Pacific Preeclampsia Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Test Type, 2017–2031
12.2.1. Blood Test
12.2.2. Urine Test
12.2.3. Others
12.3. Market Value Forecast, by Product & Services, 2017–2031
12.3.1. Instruments
12.3.2. Reagents & Consumables
12.3.3. Services
12.4. Market Value Forecast, by End-user, 2017–2031
12.4.1. Hospitals
12.4.2. Diagnostic Centers
12.4.3. Others
12.5. Market Value Forecast, by Country/Sub-Region, 2017–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Test Type
12.6.2. By Product & Services
12.6.3. By End-user
12.6.4. By Country/Sub-Region
13. Latin America Preeclampsia Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Test Type, 2017–2031
13.2.1. Blood Test
13.2.2. Urine Test
13.2.3. Others
13.3. Market Value Forecast, by Product & Services, 2017–2031
13.3.1. Instruments
13.3.2. Reagents & Consumables
13.3.3. Services
13.4. Market Value Forecast, by End-user, 2017–2031
13.4.1. Hospitals
13.4.2. Diagnostic Centers
13.4.3. Others
13.5. Market Value Forecast, by Country/Sub-Region, 2017–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Test Type
13.6.2. By Product & Services
13.6.3. By End-user
13.6.4. By Country/Sub-Region
14. Middle East & Africa Preeclampsia Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Test Type, 2017–2031
14.2.1. Blood Test
14.2.2. Urine Test
14.2.3. Others
14.3. Market Value Forecast, by Product & Services, 2017–2031
14.3.1. Instruments
14.3.2. Reagents & Consumables
14.3.3. Services
14.4. Market Value Forecast, by End-user, 2017–2031
14.4.1. Hospitals
14.4.2. Diagnostic Centers
14.4.3. Others
14.5. Market Value Forecast, by Country/Sub-Region, 2017–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Test Type
14.6.2. By Product & Services
14.6.3. By End-user
14.6.4. By Country/Sub-Region
15. Competition Landscape
15.1. Market Player – Competition Matrix (By Tier and Size of companies)
15.2. Company Profiles
15.2.1. Abbott Laboratories
15.2.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.1.2. Product Portfolio
15.2.1.3. Financial Overview
15.2.1.4. SWOT Analysis
15.2.1.5. Strategic Overview
15.2.2. Danaher Corporation (Beckman Coulter, Inc.)
15.2.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.2.2. Product Portfolio
15.2.2.3. Financial Overview
15.2.2.4. SWOT Analysis
15.2.2.5. Strategic Overview
15.2.3. Siemens Healthineers AG
15.2.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.3.2. Product Portfolio
15.2.3.3. Financial Overview
15.2.3.4. SWOT Analysis
15.2.3.5. Strategic Overview
15.2.4. Cardinal Health
15.2.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.4.2. Product Portfolio
15.2.4.3. Financial Overview
15.2.4.4. SWOT Analysis
15.2.4.5. Strategic Overview
15.2.5. F. Hoffmann-La Roche Ltd.
15.2.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.5.2. Product Portfolio
15.2.5.3. Financial Overview
15.2.5.4. SWOT Analysis
15.2.5.5. Strategic Overview
15.2.6. Bio-Rad Laboratories, Inc.
15.2.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.6.2. Product Portfolio
15.2.6.3. Financial Overview
15.2.6.4. SWOT Analysis
15.2.6.5. Strategic Overview
15.2.7. ARKRAY, Inc.
15.2.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.7.2. Product Portfolio
15.2.7.3. Financial Overview
15.2.7.4. SWOT Analysis
15.2.7.5. Strategic Overview
15.2.8. Sera Prognostics
15.2.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.8.2. Product Portfolio
15.2.8.3. Financial Overview
15.2.8.4. SWOT Analysis
15.2.8.5. Strategic Overview
15.2.9. Metabolomic Diagnostics Ltd.
15.2.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.9.2. Product Portfolio
15.2.9.3. Financial Overview
15.2.9.4. SWOT Analysis
15.2.9.5. Strategic Overview
15.2.10. Diabetomics, Inc.
15.2.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.10.2. Product Portfolio
15.2.10.3. Financial Overview
15.2.10.4. SWOT Analysis
15.2.10.5. Strategic Overview
15.2.11. ACON Laboratories, Inc.
15.2.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.11.2. Product Portfolio
15.2.11.3. Financial Overview
15.2.11.4. SWOT Analysis
15.2.11.5. Strategic Overview
15.2.12. DIRUI Industrial Co., Ltd.
15.2.12.1. Company Overview (HQ, Business Segments, Employee Strength)
15.2.12.2. Product Portfolio
15.2.12.3. Financial Overview
15.2.12.4. SWOT Analysis
15.2.12.5. Strategic Overview
List of Tables
Table 01: Global Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 02: Global Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 03: Global Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 04: Global Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 05: North America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 06: North America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 07: North America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 08: North America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 09: Europe Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 10: Europe Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 11: Europe Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 12: Europe Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 13: Asia Pacific Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 14: Asia Pacific Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 15: Asia Pacific Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 16: Asia Pacific Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 17: Latin America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 18: Latin America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 19: Latin America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 20: Latin America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 21: Middle East & Africa Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017‒2031
Table 22: Middle East & Africa Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Test Type, 2017‒2031
Table 23: Middle East & Africa Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by Product & Services, 2017–2031
Table 24: Middle East & Africa Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Global Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Preeclampsia Diagnostics Market Value Share, by Test Type, 2021
Figure 03: Preeclampsia Diagnostics Market Value Share, By Product & Services, 2021
Figure 04: Preeclampsia Diagnostics Market Value Share, by End-user 2021
Figure 05: Global Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 06: Global Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 07: Global Preeclampsia Diagnostics Market Value (US$ Mn), by Blood Test, 2017‒2031
Figure 08: Global Preeclampsia Diagnostics Market Value (US$ Mn), by Urine Test, 2017‒2031
Figure 09: Global Preeclampsia Diagnostics Market Value (US$ Mn), by Others, 2017‒2031
Figure 10: Global Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 11: Global Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 12: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Instruments, 2017–2031
Figure 13: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Reagents & Consumables, 2017–2031
Figure 14: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Services, 2017–2031
Figure 15: Global Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 16: Global Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 17: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Hospitals, 2017–2031
Figure 18: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Diagnostic Centers, 2017–2031
Figure 19: Global Preeclampsia Diagnostics Market Revenue (US$ Mn), by Others, 2017–2031
Figure 20: Global Preeclampsia Diagnostics Market Value Share Analysis, by Region, 2021 and 2031
Figure 21: Global Preeclampsia Diagnostics Market Attractiveness Analysis, by Region, 2022–2031
Figure 22: North America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 23: North America Preeclampsia Diagnostics Market Value Share Analysis, by Country, 2021 and 2031
Figure 24: North America Preeclampsia Diagnostics Market Attractiveness Analysis, by Country, 2022–2031
Figure 25: North America Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 26: North America Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 27: North America Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 28: North America Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 29: North America Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 30: North America Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 31: Europe Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 32: Europe Preeclampsia Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 33: Europe Preeclampsia Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 34: Europe Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 35: Europe Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 36: Europe Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 37: Europe Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 38: Europe Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 39: Europe Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 40: Asia Pacific Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 41: Asia Pacific Preeclampsia Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 42: Asia Pacific Preeclampsia Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 43: Asia Pacific Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 44: Asia Pacific Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 45: Asia Pacific Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 46: Asia Pacific Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 47: Asia Pacific Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 48: Asia Pacific Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 49: Latin America Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 50: Latin America Preeclampsia Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 51: Latin America Preeclampsia Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2022-2031
Figure 52: Latin America Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 53: Latin America Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 54: Latin America Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 55: Latin America Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 56: Latin America Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 57: Latin America Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 58: Middle East & Africa Preeclampsia Diagnostics Market Value (US$ Mn) Forecast, 2017–2031
Figure 59: Middle East & Africa Preeclampsia Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 60: Middle East & Africa Preeclampsia Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 61: Middle East & Africa Preeclampsia Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 62: Middle East & Africa Preeclampsia Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 63: Middle East & Africa Preeclampsia Diagnostics Market Value Share Analysis, By Product & Services, 2021 and 2031
Figure 64: Middle East & Africa Preeclampsia Diagnostics Market Attractiveness Analysis, By Product & Services, 2022–2031
Figure 65: Middle East & Africa Preeclampsia Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 66: Middle East & Africa Preeclampsia Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031