Analysts’ Viewpoint on Market Scenario
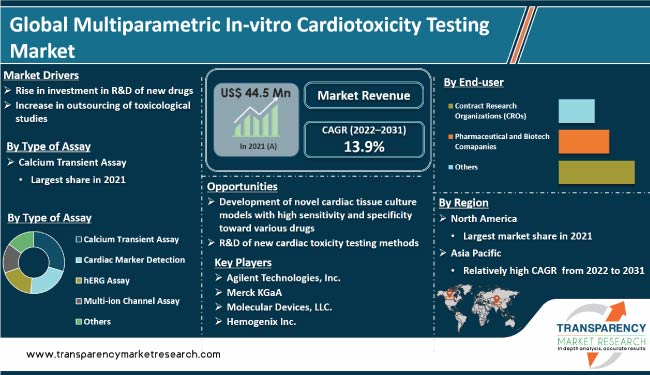
The global multiparametric in-vitro cardiotoxicity testing market size is expected to grow at a rapid pace from 2022 to 2031, led by the rise in demand for effective and affordable techniques to assess the safety of drugs. Multiparametric in-vitro cardiotoxicity testing is used to evaluate the possible toxic effects of chemicals and other compounds on the heart.
In-vitro testing can be a suitable alternative for conventional approaches employed to determine the safety of various pharmaceuticals. Implementation of stringent regulatory policies to test drug safety and surge in need for safe pharmaceutical drugs are anticipated to propel multiparametric in-vitro cardiotoxicity testing industry statistics during the forecast period.
Multiparametric in-vitro cardiotoxicity test is performed using cells or tissue culture systems in a laboratory setting, rather than in living organisms. It is used to assess the safety of a wide range of substances, including pharmaceuticals, industrial chemicals, and consumer products.
Multiparametric in-vitro cardiotoxicity tests can be conducted on tissue samples or organoids, primary cells, established cell lines, or cell cultures (three-dimensional tissue cultures that mimic the structure and function of organs). These tests are cost-effective and efficient alternatives to animal testing.
Various pharmaceutical companies are investing significantly in R&D of new therapies due to the rise in prevalence of chronic diseases. They are seeking more reliable and accurate methods to determine the efficacy and safety of these therapies.
In-vitro cardiotoxicity testing uses cell- or tissue-based models to study the potential effects of a drug on the heart. It plays an important role in assessing the risks of new medications, particularly those designed for the treatment of cardiovascular disorders. Thus, high incidence of cardiovascular diseases is estimated to contribute to multiparametric in-vitro cardiotoxicity testing market growth in the next few years.
Key pharmaceutical companies are investing in multiparametric in-vitro cardiotoxicity testing to determine the potential risks of new treatments. This gives in-depth insights into the development and commercialization of potential drugs. Investment in drug discovery has witnessed rapid growth in the recent past due to the rise in cases of chronic and infectious diseases. This, in turn, is likely to drive the multiparametric in-vitro cardiotoxicity testing market revenue in the near future.
Various stakeholders in the life sciences sector are opting for strategic alliances with Contract Research Organizations (CROs). Biopharmaceutical companies are under intense pressure to reduce the cost of drug discovery and development. Therefore, these companies prefer to outsource R&D activities to CROs. They also outsource non-core services, such as toxicity research, thereby augmenting multiparametric in-vitro cardiotoxicity testing market development.
Toxicological studies have become more sophisticated and require extensive experience in toxicological testing. Outsourcing toxicological studies can help address the limitations of in-house toxicological research, such as a lack of skilled labor, dearth of sophisticated tools and technologies, and lack of expertise in regulatory standards. This is likely to boost market expansion in the next few years.
According to the latest multiparametric in-vitro cardiotoxicity testing market analysis, the calcium transient assay segment accounted for the largest share in 2021. Calcium transient tests are used to measure calcium concentration. The ability of muscle cells to contract, particularly cardiomyocytes, plays an important role in determining compound-induced cardiotoxicity.
Calcium transient assays provide valuable information about the effects of a substance on the function of heart cells. Calcium ions play a critical role in the contraction of heart muscle cells. Changes in concentration of calcium ions inside the cells can indicate potential disruptions in normal heart function.
The Contract Research Organizations (CROs) end-user segment dominated the market in 2021. Outsourcing in-vitro cardiotoxicity testing to a CRO can be more cost-effective than conducting the testing in-house.
CROs possess specialized equipment and trained personnel. They can often complete the testing more efficiently and at a lower cost than a company can with less experience in the field. CROs often have the capacity to handle large volumes of testing and can provide rapid turnaround times for results.
North America is projected to hold the largest share of the industry during the forecast period. Rise in need for safe and high-quality cosmetic products is likely to boost the multiparametric in-vitro cardiotoxicity testing market share of the region in the next few years. Several stakeholders in healthcare and pharmaceutical sectors in North America are collaborating with CROs for preclinical product testing.
The market in Europe is expected to grow at a significant pace during the forecast period. Increase in prevalence of cardiovascular diseases and surge in investment in drug discovery and development are driving market progress in the region.
The market in Asia Pacific is driven by the rise in cost of preclinical and clinical testing in the region. Pharmaceutical companies in developing countries, such as China, India, and Japan, are partnering with CROs to obtain rapid outcomes in drug testing at low costs.
Increase in investment in healthcare and pharmaceutical sectors and rise in consumer awareness about the safety of drugs are likely to fuel market statistics in Rest of the World (Latin America and Middle East & Africa) during the forecast period.
The market report profiles various vendors based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments. The global industry is fragmented, with the presence of a large number of leading service providers.
Creative Bioarray, Agilent Technologies, Inc., Hemogenix Inc., Merck KGaA, Molecular Devices, LLC., Evotec, Miltenyi Biotec, FUJIFILM Cellular Dynamics, Enzo Life Sciences, Inc., Axol Bioscience Ltd., Stemina Biomarker Discovery, Inc., Eurofins Discovery, and emka TECHNOLOGIES are prominent companies operating in this market.
|
Attribute |
Detail |
|
Size in 2021 |
US$ 44.5 Mn |
|
Forecast Value in 2031 |
More than US$ 166.5 Mn |
|
CAGR - 2022–2031 |
13.9% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2017–2021 |
|
Quantitative Units |
US$ Mn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
.Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon Request |
|
Pricing |
Available upon Request |
It was valued at US$ 44.5 Mn in 2021.
It is projected to reach more than US$ 166.5 Mn by 2031.
It is anticipated to be 13.9% from 2022 to 2031.
The Contract Research Organizations (CROs) segment held more than 45.0% share in 2021.
North America is expected to account for the largest share from 2022 to 2031.
Creative Bioarray, Agilent Technologies, Inc., Hemogenix Inc., Merck KGaA, Molecular Devices, LLC., Evotec, Miltenyi Biotec, FUJIFILM Cellular Dynamics, Enzo Life Sciences, Inc., Axol Bioscience Ltd., Stemina Biomarker Discovery, Inc., Eurofins Discovery, and emka TECHNOLOGIES.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Multiparametric In-vitro Cardiotoxicity Testing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast, 2017 - 2031
4.4.1. Market Revenue Projection (US$ Mn)
5. Key Insights
5.1. Key Industry Developments
5.2. Key Product/Brand Analysis
5.3. Emerging Technologies and Platforms for Cardiotoxicity Testing
5.4. Overview of In-vitro Cardiac Electrophysiology Platforms for Toxicology Assessment
5.5. Overview of Multiparametric In-vitro Toxicology Assessment
5.6. COVID-19 Impact Analysis
6. Global Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast, By Type of Assay
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast By Type of Assay, 2017 - 2031
6.3.1. Calcium Transient Assay
6.3.2. Cardiac Marker Detection
6.3.3. hERG Assay
6.3.4. Multi-ion Channel Assay
6.3.5. Others
6.4. Market Attractiveness By Type of Assay
7. Global Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast, By End-user
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast By End-user, 2017 - 2031
7.3.1. Contract Research Organizations (CROs)
7.3.2. Pharmaceutical and Biotech Companies
7.3.3. Others
7.4. Market Attractiveness By End-user
8. Global Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast, By Region
8.1. Key Findings
8.2. Market Value Forecast By Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Rest of the World
8.3. Market Attractiveness By Country/Region
9. North America Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast By Type of Assay, 2017 - 2031
9.2.1. Calcium Transient Assay
9.2.2. Cardiac Marker Detection
9.2.3. hERG Assay
9.2.4. Multi-ion Channel Assay
9.2.5. Others
9.3. Market Value Forecast By End-user, 2017 - 2031
9.3.1. Contract Research Organizations (CROs)
9.3.2. Pharmaceutical and Biotech Companies
9.3.3. Others
9.4. Market Value Forecast By Country, 2017 - 2031
9.4.1. U.S.
9.4.2. Canada
9.5. Market Attractiveness Analysis
9.5.1. By Type of Assay
9.5.2. By End-user
9.5.3. By Country
10. Europe Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast By Type of Assay, 2017 - 2031
10.2.1. Calcium Transient Assay
10.2.2. Cardiac Marker Detection
10.2.3. hERG Assay
10.2.4. Multi-ion Channel Assay
10.2.5. Others
10.3. Market Value Forecast By End-user, 2017 - 2031
10.3.1. Contract Research Organizations (CROs)
10.3.2. Pharmaceutical and Biotech Companies
10.3.3. Others
10.4. Market Value Forecast By Country/Sub-region, 2017 - 2031
10.4.1. Germany
10.4.2. U.K.
10.4.3. France
10.4.4. Italy
10.4.5. Spain
10.4.6. Rest of Europe
10.5. Market Attractiveness Analysis
10.5.1. By Type of Assay
10.5.2. By End-user
10.5.3. By Country/Sub-region
11. Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast By Type of Assay, 2017 - 2031
11.2.1. Calcium Transient Assay
11.2.2. Cardiac Marker Detection
11.2.3. hERG Assay
11.2.4. Multi-ion Channel Assay
11.2.5. Others
11.3. Market Value Forecast By End-user, 2017 - 2031
11.3.1. Contract Research Organizations (CROs)
11.3.2. Pharmaceutical and Biotech Companies
11.3.3. Others
11.4. Market Value Forecast By Country/Sub-region, 2017 - 2031
11.4.1. China
11.4.2. Japan
11.4.3. India
11.4.4. Australia & New Zealand
11.4.5. Rest of Asia Pacific
11.5. Market Attractiveness Analysis
11.5.1. By Type of Assay
11.5.2. By End-user
11.5.3. By Country/Sub-region
12. Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast By Type of Assay, 2017 - 2031
12.2.1. Calcium Transient Assay
12.2.2. Cardiac Marker Detection
12.2.3. hERG Assay
12.2.4. Multi-ion Channel Assay
12.2.5. Others
12.3. Market Value Forecast By End-user, 2017 - 2031
12.3.1. Contract Research Organizations (CROs)
12.3.2. Pharmaceutical and Biotech Companies
12.3.3. Others
12.4. Market Attractiveness Analysis
12.4.1. By Type of Assay
12.4.2. By End-user
13. Competition Landscape
13.1. Market Player – Competition Matrix (By Tier and Size of Companies)
13.2. Market Share Analysis By Company (2021)
13.3. Company Profiles
13.3.1. Creative Bioarray
13.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.1.2. Product Portfolio
13.3.1.3. SWOT Analysis
13.3.1.4. Strategic Overview
13.3.2. Agilent Technologies, Inc.
13.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.2.2. Product Portfolio
13.3.2.3. SWOT Analysis
13.3.2.4. Strategic Overview
13.3.3. Hemogenix Inc.
13.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.3.2. Product Portfolio
13.3.3.3. SWOT Analysis
13.3.3.4. Strategic Overview
13.3.4. Merck KGaA
13.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.4.2. Product Portfolio
13.3.4.3. SWOT Analysis
13.3.4.4. Strategic Overview
13.3.5. Molecular Devices, LLC
13.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.5.2. Product Portfolio
13.3.5.3. SWOT Analysis
13.3.5.4. Strategic Overview
13.3.6. Evotec
13.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.6.2. Product Portfolio
13.3.6.3. SWOT Analysis
13.3.6.4. Strategic Overview
13.3.7. Miltenyi Biotec
13.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.7.2. Product Portfolio
13.3.7.3. SWOT Analysis
13.3.7.4. Strategic Overview
13.3.8. FUJIFILM Cellular Dynamics
13.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.8.2. Product Portfolio
13.3.8.3. SWOT Analysis
13.3.8.4. Strategic Overview
13.3.9. Enzo Life Sciences, Inc.
13.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.9.2. Product Portfolio
13.3.9.3. SWOT Analysis
13.3.9.4. Strategic Overview
13.3.10. Axol Bioscience Ltd.
13.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.10.2. Product Portfolio
13.3.10.3. SWOT Analysis
13.3.10.4. Strategic Overview
13.3.11. Stemina Biomarker Discovery, Inc.
13.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.11.2. Product Portfolio
13.3.11.3. SWOT Analysis
13.3.11.4. Strategic Overview
13.3.12. emka TECHNOLOGIES
13.3.12.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.12.2. Product Portfolio
13.3.12.3. SWOT Analysis
13.3.12.4. Strategic Overview
13.3.13. Eurofins Discovery
13.3.13.1. Company Overview (HQ, Business Segments, Employee Strength)
13.3.13.2. Product Portfolio
13.3.13.3. SWOT Analysis
13.3.13.4. Strategic Overview
List of Tables
Table 01: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Type of Assay, 2017‒2031
Table 02: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 03: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 04: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 05: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Type of Assay, 2017‒2031
Table 06: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 07: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Country/Sub-Region, 2017–2031
Table 08: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Type of Assay, 2017‒2031
Table 09: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 10: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 11: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Type of Assay, 2017‒2031
Table 12: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 13: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by Type of Assay, 2017‒2031
Table 14: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Multiparametric In-vitro Cardiotoxicity Testing Market Value Share, by Type of Assay, 2021
Figure 03: Multiparametric In-vitro Cardiotoxicity Testing Market Value Share, by End-user, 2021
Figure 04: Multiparametric In-vitro Cardiotoxicity Testing Market Value Share, by Region, 2021
Figure 05: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Type of Assay, 2021 and 2031
Figure 06: Global Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Type of Assay, 2022–2031
Figure 07: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn), by Calcium Transient Assay, 2017‒2031
Figure 08: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn), by Cardiac Marker Detection, 2017‒2031
Figure 09: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn), by hERG Assay, 2017‒2031
Figure 10: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn), by Multi-ion Channel Assay, 2017‒2031
Figure 11: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn), by others, 2017‒2031
Figure 12: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by End-user, 2021 and 2031
Figure 13: Global Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by End-user, 2022–2031
Figure 14: Global Multiparametric In-vitro Cardiotoxicity Testing Market Revenue (US$ Mn), by Contract Research Organizations (CROs), 2017–2031
Figure 15: Global Multiparametric In-vitro Cardiotoxicity Testing Market Revenue (US$ Mn), by Pharmaceutical and Biotech Companies, 2017–2031
Figure 16: Global Multiparametric In-vitro Cardiotoxicity Testing Market Revenue (US$ Mn), by others, 2017–2031
Figure 17: Global Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Region, 2021 and 2031
Figure 18: Global Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Region, 2022–2031
Figure 19: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 20: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Country, 2021 and 2031
Figure 21: North America Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Country, 2022–2031
Figure 22: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Type of Assay, 2021 and 2031
Figure 23: North America Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Type of Assay 2022–2031
Figure 24: North America Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by End-user, 2021 and 2031
Figure 25: North America Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by End-user, 2022–2031
Figure 26: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 27: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 28: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 29: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Type of Assay, 2021 and 2031
Figure 30: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Type of Assay 2022–2031
Figure 31: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by End-user, 2021 and 2031
Figure 32: Europe Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by End-user, 2022–2031
Figure 33: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 34: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 35: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Country/Sub-region, 2022–2031
Figure 36: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Type of Assay, 2021 and 2031
Figure 37: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Type of Assay 2022–2031
Figure 38: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by End-user, 2021 and 2031
Figure 39: Asia Pacific Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by End-user, 2022–2031
Figure 40: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Value (US$ Mn) Forecast, 2017–2031
Figure 41: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by Type of Assay, 2021 and 2031
Figure 42: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by Type of Assay 2022–2031
Figure 43: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Value Share Analysis, by End-user, 2021 and 2031
Figure 44: Rest of the World Multiparametric In-vitro Cardiotoxicity Testing Market Attractiveness Analysis, by End-user, 2022–2031
Figure 45: Company Share Analysis, 2021