Analysts’ Viewpoint
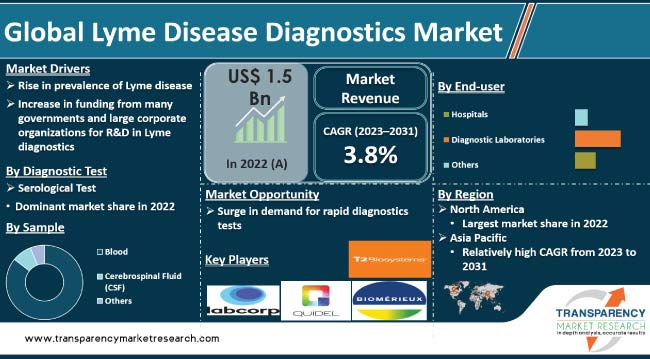
Rise in incidence of the Lyme disease and growth in awareness about diagnostic tests are key Lyme disease diagnostics market drivers. The number of people affected with the Lyme disease is rising, particularly in regions with high prevalence of ticks. Development of advanced diagnostic technologies, such as nucleic acid amplification tests (NAATs), enzyme immunoassays (EIAs), and multiplex assays, has improved the accuracy and efficiency of Lyme disease diagnosis. Availability of innovative diagnostic tools is expected to boost market statistics in the near future.
Increase in funding from several governments and large corporate organizations for R&D in the field of Lyme disease diagnostics is contributing to market growth. Advancements in the field are anticipated to enhance accuracy, sensitivity, and specificity of Lyme disease testing, thereby creating lucrative opportunities for test providers.
Lyme disease diagnostics is a rapidly growing market in the healthcare industry. Lyme disease, caused by the bacteria Borrelia burgdorferi, is a tick-borne illness that spreads to humans through the infected blacklegged ticks. Typical symptoms include fever, headache, tiredness, and skin rash, which can have serious health implications if left untreated.
Prevalence of the Lyme disease has been rising, especially in regions with high tick populations. This has led to an increase in awareness about the disease and need for early detection and treatment. Demand for accurate and efficient diagnostic tools is rising across the globe owing to the growth in incidence of the Lyme disease.
According to the CDC, confirmed cases of the Lyme disease in the U.S. rose to 44.0% from 1999 to 2019. Lyme disease is more prevalent in North America and Europe. According to an article published by BMJ Global Health (June 2022), more than 14.0% of the world’s population is suffering from the tick-borne Lyme disease, indicated by the presence of antibodies in the blood.
There are just a few accurate tests for diagnosis of the Lyme disease. Furthermore, continual mutations and genotypic variations in the bacteria are augmenting the chances of their prevalence among the population across the globe.
Rise in incidence of the disease is encouraging research and development activities in the field of Lyme disease detection technology. As a result, the global Lyme disease diagnostics market is experiencing steady growth.
The serological diagnostic test segment is projected to dominate the global landscape during the forecast period. Serological test is an initial test performed in most cases of suspected Lyme disease. It detects antibodies that the body produces in response to the infection.
Serological test is the most affordable option for Lyme disease diagnosis. ELISA and western blot are the commonly used serological tests. ELISA accounted for the larger market share in 2022. ELISA is known for its high sensitivity in detecting antibodies against Borrelia burgdorferi, the bacteria that causes Lyme disease.
Affordability and widespread availability of ELISA kits and rapid turnaround time for prompt detection have led to high utilization of the diagnostic test in hospitals and other healthcare settings.
The blood sample segment held the largest share of the global Lyme disease diagnostics market in 2022. The trend is expected to continue during the forecast period.
Blood samples are increasingly used to detect the Lyme disease. In Lyme disease tests, physicians are able to visualize the reaction between antibodies in an infected person's blood to specific antigens that cause the disease.
Blood sample can be drawn easily in a doctor’s office or other medical settings. These samples are analyzed with enzyme immunoassay, immunofluorescence assay, or the western blot method for the Lyme disease. For instance, the CDC-approved two-tiered serologic test for Lyme disease is carried out via the blood sample.
The diagnostic laboratories end-user segment accounted for significant market share in 2022. The segment is projected to dominate during the next few years.
Diagnostic labs typically have lower overhead costs than hospitals. They offer Lyme disease tests at more affordable prices, making them more accessible to patients. Diagnostic laboratories adhere to stringent quality assurance protocols to ensure the accuracy and reliability of Lyme disease diagnostic tests.
Diagnostic laboratories also collaborate with research institutes and industry partners to drive innovation in Lyme disease diagnostics. Such collaborations facilitate the development of new diagnostic technologies, improvement of existing testing methods, and validation of novel assays. Diagnostic laboratories are gaining traction due to faster turnaround times, diagnostic testing expertise, and convenience to patients in terms of location and time.
According to the latest Lyme disease diagnostics market forecast, North America is likely to constitute large share of the global industry in the near future. Increase in incidence of the Lyme disease, specifically in the U.S., and presence of a well-established healthcare system are contributing to the Lyme disease diagnostics market growth in the region. Latest advancements in Lyme disease diagnostics in the U.S. are also fueling market dynamics of North America.
Rise in awareness about Lyme disease screening among healthcare professionals and the general population is leading to more proactive testing and early detection of the disease. North America also has a robust diagnostic infrastructure and easy availability of advanced diagnostic tools and assays. This is accelerating Lyme disease diagnostics market development in the region.
The Lyme disease diagnostics market size in Asia Pacific is likely to increase at a steady pace during the forecast period, owing to expansion in the healthcare infrastructure, rise in prevalence of the Lyme disease, growth in R&D efforts, and surge in government initiatives to promote screening programs in the region.
The global landscape is fragmented, with the presence of a few leading players that control majority of the Lyme disease diagnostics market share. As per the latest Lyme disease diagnostics market analysis, these players are involved in mergers & acquisitions, partnerships, strategic collaborations, and new product launches to strengthen their position.
Some of the key players operating in the global market are LabCorp, Quest Diagnostics, Abbott Laboratories, Bio-Rad Laboratories, Inc., bioMérieux SA, DiaSorin S.p.A, T2 Biosystems, Inc., F. Hoffmann-La Roche AG, Meridian Bioscience, Inc., Quidel Corporation, and Thermo Fisher Scientific Inc. These companies are following the latest Lyme disease diagnostics market trends to avail lucrative growth opportunities.
Prominent players have been profiled in the Lyme disease diagnostics market report based on parameters such as product portfolio, company overview, recent developments, financial overview, business strategies, and business segments.
| Attribute | Detail |
|---|---|
|
Market Value in 2022 |
US$ 1.5 Bn |
|
Market Forecast Value in 2031 |
More than US$ 2.1 Bn |
|
Growth Rate (CAGR) |
3.8% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2021 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
It was valued at US$ 1.5 Bn in 2022
It is projected to reach more than US$ 2.1 Bn 2031
The CAGR is anticipated to be 3.8% from 2023 to 2031
Rise in prevalence of Lyme disease and increase in funding from several governments and large corporate organizations for R&D in Lyme diagnostics
The serological test segment accounted for more than 75.0% share in 2022
North America is anticipated to constitute significant share from 2023 to 2031
LabCorp, Quest Diagnostics, Abbott Laboratories, Bio-Rad Laboratories, Inc., bioMérieux SA, DiaSorin S.p.A., T2 Biosystems, Inc., F. Hoffmann-La Roche AG, Meridian Bioscience, Inc., Quidel Corporation, and Thermo Fisher Scientific Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Lyme Disease Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Lyme Disease Diagnostics Market Analysis and Forecast, 2017-2031
4.4.1. Market Revenue Projection (US$ Mn)
5. Key Insights
5.1. Innovations in Diagnostic Testing for Lyme disease
5.2. Incidence and Prevalence of Lyme Disease
5.3. Regulatory Scenario by Region/Globally
5.4. COVID-19 Pandemic Impact on Industry (Value Chain and Short/Mid/Long Term Impact)
6. Global Lyme Disease Diagnostics Market Analysis and Forecast, by Diagnostic Test
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Diagnostic Test, 2017-2031
6.3.1. Serological Test
6.3.1.1. ELISA
6.3.1.2. Western Blot
6.3.2. Polymerase Chain Reaction (PCR) test
6.3.3. Others
6.4. Market Attractiveness Analysis, by Diagnostic Test
7. Global Lyme Disease Diagnostics Market Analysis and Forecast, by Sample
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Sample, 2017-2031
7.3.1. Blood
7.3.2. Cerebrospinal Fluid (CSF)
7.3.3. Others
7.4. Market Attractiveness Analysis, by Sample
8. Global Lyme Disease Diagnostics Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by End-user, 2017-2031
8.3.1. Hospitals
8.3.2. Diagnostic Laboratories
8.3.3. Others
8.4. Market Attractiveness Analysis, by End-user
9. Global Lyme Disease Diagnostics Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2017-2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Lyme Disease Diagnostics Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Diagnostic Test, 2017-2031
10.2.1. Serological Test
10.2.1.1. ELISA
10.2.1.2. Western Blot
10.2.2. Polymerase Chain Reaction (PCR) test
10.2.3. Others
10.3. Market Value Forecast, by Sample, 2017-2031
10.3.1. Blood
10.3.2. Cerebrospinal Fluid (CSF)
10.3.3. Others
10.4. Market Value Forecast, by End-user, 2017-2031
10.4.1. Hospitals
10.4.2. Diagnostic Laboratories
10.4.3. Others
10.5. Market Value Forecast, by Country, 2017-2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Diagnostic Test
10.6.2. By Sample
10.6.3. By End-user
10.6.4. By Country
11. Europe Lyme Disease Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Diagnostic Test, 2017-2031
11.2.1. Serological Test
11.2.1.1. ELISA
11.2.1.2. Western Blot
11.2.2. Polymerase Chain Reaction (PCR) test
11.2.3. Others
11.3. Market Value Forecast, by Sample, 2017-2031
11.3.1. Blood
11.3.2. Cerebrospinal Fluid (CSF)
11.3.3. Others
11.4. Market Value Forecast, by End-user, 2017-2031
11.4.1. Hospitals
11.4.2. Diagnostic Laboratories
11.4.3. Others
11.5. Market Value Forecast, by Country/Sub-region, 2017-2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Diagnostic Test
11.6.2. By Sample
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Lyme Disease Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Diagnostic Test, 2017-2031
12.2.1. Serological Test
12.2.1.1. ELISA
12.2.1.2. Western Blot
12.2.2. Polymerase Chain Reaction (PCR) test
12.2.3. Others
12.3. Market Value Forecast, by Sample, 2017-2031
12.3.1. Blood
12.3.2. Cerebrospinal Fluid (CSF)
12.3.3. Others
12.4. Market Value Forecast, by End-user, 2017-2031
12.4.1. Hospitals
12.4.2. Diagnostic Laboratories
12.4.3. Others
12.5. Market Value Forecast, by Country/Sub-region, 2017-2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Diagnostic Test
12.6.2. By Sample
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Lyme Disease Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Diagnostic Test, 2017-2031
13.2.1. Serological Test
13.2.1.1. ELISA
13.2.1.2. Western Blot
13.2.2. Polymerase Chain Reaction (PCR) test
13.2.3. Others
13.3. Market Value Forecast, by Sample, 2017-2031
13.3.1. Blood
13.3.2. Cerebrospinal Fluid (CSF)
13.3.3. Others
13.4. Market Value Forecast, by End-user, 2017-2031
13.4.1. Hospitals
13.4.2. Diagnostic Laboratories
13.4.3. Others
13.5. Market Value Forecast, by Country/Sub-region, 2017-2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Diagnostic Test
13.6.2. By Sample
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Lyme Disease Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Diagnostic Test, 2017-2031
14.2.1. Serological Test
14.2.1.1. ELISA
14.2.1.2. Western Blot
14.2.2. Polymerase Chain Reaction (PCR) test
14.2.3. Others
14.3. Market Value Forecast, by Sample, 2017-2031
14.3.1. Blood
14.3.2. Cerebrospinal Fluid (CSF)
14.3.3. Others
14.4. Market Value Forecast, by End-user, 2017-2031
14.4.1. Hospitals
14.4.2. Diagnostic Laboratories
14.4.3. Others
14.5. Market Value Forecast, by Country/Sub-region, 2017-2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Diagnostic Test
14.6.2. By Sample
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competition Matrix (By Tier and Size of Companies)
15.2. Market Share Analysis, by Company (2022)
15.3. Company Profiles
15.3.1. LabCorp
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.1.5. Strategic Overview
15.3.2. Quest Diagnostics
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. Abbott Laboratories
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Financial Overview
15.3.3.4. SWOT Analysis
15.3.3.5. Strategic Overview
15.3.4. Bio-Rad Laboratories, Inc.
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. bioMérieux SA
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.5.5. Strategic Overview
15.3.6. DiaSorin S.p.A.
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.6.5. Strategic Overview
15.3.7. T2 Biosystems, Inc.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.7.5. Strategic Overview
15.3.8. F. Hoffmann-La Roche AG
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.8.5. Strategic Overview
15.3.9. Meridian Bioscience, Inc.
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Financial Overview
15.3.9.4. SWOT Analysis
15.3.9.5. Strategic Overview
15.3.10. Quidel Corporation
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. Financial Overview
15.3.10.4. SWOT Analysis
15.3.10.5. Strategic Overview
List of Tables
Table 01: Global Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic Test, 2017-2031
Table 02: Global Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2032
Table 03: Global Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2031
Table 04: Global Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2032
Table 05: Global Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 06: North America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic Test, 2017-2031
Table 07: North America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2031
Table 08: North America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2032
Table 09: North America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2031
Table 10: North America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 11: Europe Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic Test, 2017-2031
Table 12: Europe Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2031
Table 13: Europe Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2031
Table 14: Europe Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2031
Table 15: Europe Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 16: Asia Pacific Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic Test, 2017-2031
Table 17: Asia Pacific Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2031
Table 18: Asia Pacific Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2031
Table 19: Asia Pacific Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2031
Table 20: Asia Pacific Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 21: Latin America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic Test, 2017-2031
Table 22: Latin America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2031
Table 23: Latin America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2031
Table 24: Latin America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2031
Table 25: Latin America Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
Table 26: Middle East & Africa Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Diagnostic test, 2017-2031
Table 27: Middle East & Africa Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Serological Test, 2017-2031
Table 28: Middle East & Africa Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Sample, 2017-2031
Table 29: Middle East & Africa Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2017-2031
Table 30: Middle East & Africa Lyme Disease Diagnostics Market Size (US$ Mn) Forecast, by Region, 2017-2031
List of Figures
Figure 01: Global Lyme Disease Diagnostics Market Value (US$ Mn) Forecast, 2017-2031
Figure 02: Global Lyme Disease Diagnostics Market Value Share, by Diagnostic Test, 2022
Figure 03: Global Lyme Disease Diagnostics Market Value Share, by Sample, 2022
Figure 04: Global Lyme Disease Diagnostics Market Value Share, by End-user, 2022
Figure 05: Global Lyme Disease Diagnostics Market Value Share, Analysis, by Diagnostic Test, 2022 and 2031
Figure 06: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Serological Test, 2017-2031
Figure 07: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Polymerase Chain Reaction (PCR) Test, 2017-2031
Figure 08: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Others, 2017-2031
Figure 09: Global Lyme Disease Diagnostics Market Attractiveness Analysis, by Diagnostic Test, 2023-2031
Figure 10: Global Lyme Disease Diagnostics Market Value Share, Analysis, by Sample, 2022 and 2031
Figure 11: Global Lyme Disease Diagnostics Market Revenue (US$ Mn) by Blood, 2017-2031
Figure 12: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Cerebrospinal Fluid (CSF), 2017-2031
Figure 13: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Others, 2017-2031
Figure 14: Global Lyme Disease Diagnostics Market Attractiveness Analysis, by Sample, 2023-2031
Figure 15: Global Lyme Disease Diagnostics Market Value Share, Analysis, by End-user, 2022 and 2031
Figure 16: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Hospitals, 2017-2031
Figure 17: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Diagnostic Laboratories, 2017-2031
Figure 18: Global Lyme Disease Diagnostics Market Revenue (US$ Mn), by Others, 2017-2031
Figure 19: Global Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 20: Global Lyme Disease Diagnostics Market Value Share, Analysis, by Region, 2022 and 2031
Figure 21: Global Lyme Disease Diagnostics Market Attractiveness Analysis, by Region, 2023-2031
Figure 22: North America Lyme Disease Diagnostics Market Value (US$ Mn) Forecast, 2017-2031
Figure 23: North America Lyme Disease Diagnostics Market Value Share, Analysis, by Country, 2022 and 2031
Figure 24: North America Lyme Disease Diagnostics Market Attractiveness Analysis, by Country, 2023-2031
Figure 25: North America Lyme Disease Diagnostics Market Value Share, Analysis, by Diagnostic Test, 2022 and 2031
Figure 26: North America Lyme Disease Diagnostics Market Attractiveness Analysis, by Diagnostic Test, 2023-2031
Figure 27: North America Lyme Disease Diagnostics Market Value Share, Analysis, by Sample, 2022 and 2031
Figure 28: North America Lyme Disease Diagnostics Market Attractiveness Analysis, by Sample, 2023-2031
Figure 29: North America Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2032
Figure 30: North America Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 31: Europe Lyme Disease Diagnostics Market Value (US$ Mn) Forecast, 2017-2031
Figure 32: Europe Lyme Disease Diagnostics Market Value Share, Analysis, by Country, 2022 and 2031
Figure 33: Europe Lyme Disease Diagnostics Market Attractiveness Analysis, by Country, 2023-2031
Figure 34: Europe Lyme Disease Diagnostics Market Value Share, Analysis, by Diagnostic Test, 2022 and 2031
Figure 35: Europe Lyme Disease Diagnostics Market Attractiveness Analysis, by Diagnostic Test, 2023-2031
Figure 36: Europe Lyme Disease Diagnostics Market Value Share Analysis, by Sample, 2022 and 2031
Figure 37: Europe Lyme Disease Diagnostics Market Attractiveness Analysis, by Sample, 2023-2031
Figure 38: Europe Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2032
Figure 39: Europe Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 40: Asia Pacific Lyme Disease Diagnostics Market Value (US$ Mn) Forecast, 2017-2031
Figure 41: Asia Pacific Lyme Disease Diagnostics Market Value Share Analysis, by Country, 2022 and 2031
Figure 42: Asia Pacific Lyme Disease Diagnostics Market Attractiveness Analysis, by Country, 2023-2031
Figure 43: Asia Pacific Lyme Disease Diagnostics Market Value Share, Analysis, by Diagnostic Test, 2022 and 2031
Figure 44: Asia Pacific Lyme Disease Diagnostics Market Attractiveness Analysis, by Diagnostic Test, 2023-2031
Figure 45: Asia Pacific Lyme Disease Diagnostics Market Value Share, Analysis, by Sample, 2022 and 2031
Figure 46: Asia Pacific Lyme Disease Diagnostics Market Attractiveness Analysis, by Sample, 2023-2031
Figure 47: Asia Pacific Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2032
Figure 48: Asia Pacific Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 49: Latin America Lyme Disease Diagnostics Market Value (US$ Mn) Forecast, 2017-2031
Figure 50: Latin America Lyme Disease Diagnostics Market Value Share, Analysis, by Country, 2022 and 2031
Figure 51: Latin America Lyme Disease Diagnostics Market Attractiveness Analysis, by Country, 2023-2031
Figure 52: Latin America Lyme Disease Diagnostics Market Value Share, Analysis, by Diagnostic Test, 2022 and 2031
Figure 53: Latin America Lyme Disease Diagnostics Market Attractiveness Analysis, by Diagnostic Test, 2023-2031
Figure 54: Latin America Lyme Disease Diagnostics Market Value Share Analysis, by Sample, 2022 and 2031
Figure 55: Latin America Lyme Disease Diagnostics Market Attractiveness Analysis, by Sample, 2023-2031
Figure 56: Latin America Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2032
Figure 57: Latin America Lyme Disease Diagnostics Market Attractiveness Analysis, by End-user, 2023-2031
Figure 58: Middle East & Africa Lyme Disease Diagnostics Market Value (US$ Mn), 2017-2031
Figure 59: Middle East & Africa Lyme Disease Diagnostics Market Value Share Analysis, by Country, 2022 and 2031
Figure 60: Middle East & Africa Lyme Disease Diagnostics Market Attractiveness, by Country, 2023-2031
Figure 61: Middle East & Africa Lyme Disease Diagnostics Market Value Share Analysis, by Diagnostic Test, 2022 and 2031
Figure 62: Middle East & Africa Lyme Disease Diagnostics Market Attractiveness, by Diagnostic Test, 2023-2031
Figure 63: Middle East & Africa Lyme Disease Diagnostics Market Value Share Analysis, by Sample, 2022 and 2031
Figure 64: Middle East & Africa Lyme Disease Diagnostics Market Attractiveness, by Sample, 2023-2031
Figure 65: Middle East & Africa Lyme Disease Diagnostics Market Attractiveness, by End-user, 2023-2032
Figure 66: Middle East & Africa Lyme Disease Diagnostics Market Attractiveness, by End-user, 2023-2031
Figure 67: Company Share Analysis Lyme Disease Diagnostics, 2022