Analysts’ Viewpoint
Increase in number of COVID-19 cases is expected to fuel market expansion in the next few years. Usage of telemedicine and remote monitoring is likely to increase during the pandemic, thus driving the demand for home-based diagnostic tests.
Development of new diagnostic technologies is anticipated to create growth opportunities for market players. Leading players are investing significantly in development of loop-mediated isothermal amplification (LAMP), nucleic acid sequence-based amplification (NASBA), and CRISPR-based tests that can detect COVID-19 more accurately and quickly. However, decrease in demand for COVID-19 tests due to the decline in number of new cases and rise in number of people getting vaccinated are likely to be market limitations during the forecast period.
COVID-19 diagnostics are used to detect the presence of the SARS-CoV-2 virus, which causes COVID-19. The market includes a range of diagnostic tests, including RT-PCR tests, rapid antigen tests, and serological tests (antibody tests).
The COVID-19 pandemic has led to significant increase in demand for diagnostic tests to detect the virus. This has created significant opportunities for companies that develop and manufacture these tests. The market is expected to grow in the next few years led by rise in number of COVID-19 cases and development of new diagnostic technologies.
Different types of tests and services are available in the market, such as RT-PCR tests, rapid antigen tests, serological tests, and point-of-care tests. RT-PCR tests are considered ‘gold standard’ for COVID diagnosis and account for the largest share of the market. Rapid antigen tests cost less and offer faster turnaround time than RT-PCR tests. Popularity of these tests is expected to increase in the next few years.
The global market has witnessed numerous advancements in the last few years. Abbott Laboratories developed a rapid antigen test that can produce results in 15 minutes. The test is designed to be easy to carry out and could be administered in various settings, including physician offices, clinics, and at home. The test is highly accurate and has received Emergency Use Authorization from the U.S. Food and Drug Administration (FDA). Other companies, such as Roche, Thermo Fisher, LabCorp, and Quest Diagnostics, have developed PCR based-tests that are considered gold standard for COVID testing.
Lateral flow tests were also developed during the pandemic. These tests use antibodies to detect the presence of viral antigens in a sample. These are relatively fast and can produce results in 15 minutes to 30 minutes.
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) test is a new generation of rapid test developed in the last few years. It is a one-step, isothermal nucleic acid amplification assay that is able to detect the virus in less than an hour. It is highly sensitive and specific, and can be performed at point-of-care settings.
Some companies have developed portable and self-administered rapid testing devices that can produce results on the spot, enabling individuals to receive a diagnosis without having to go to a testing facility. These advancements in rapid testing technologies could help increase testing capacity and improve the speed and efficiency of COVID-19 diagnosis, which can assist in controlling the spread of the virus.
The COVID-19 pandemic has led to unprecedented changes in the healthcare system, including the diagnostic industry. The pandemic has highlighted both the industry's challenges and strengths, as well as valuable insights into how to better prepare for future pandemics.
The pandemic has demonstrated the importance of international cooperation and collaboration. Its significance is obvious and widely acknowledged. The Access to COVID-19 Tools (ACT) Accelerator announced a set of agreements to make affordable, high-quality COVID-19 antigen rapid tests available to low and middle-income countries.
The Bill & Melinda Gates Foundation, the Clinton Health Access Initiative (CHAI), the Foundation for Innovative New Diagnostics (FIND), the Global Fund, and the World Health Organization are a few of the organizations involved in the milestone agreements.
Point-of-care diagnostic solutions became a significant force for the development of molecular diagnostics during the COVID-19 pandemic. Leading diagnostic companies have embraced cutting-edge technologies with creative businesses to deliver products that could speed up and improve testing. Companies were able to introduce new COVID-19 diagnostic testing due to cooperation and partnerships, which is expected to propel the global COVID-19 diagnostics market size.
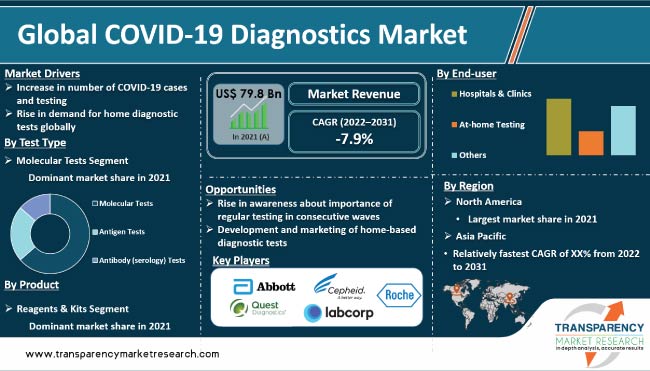
In terms of test type, the molecular tests segment is projected to account for largest global COVID-19 diagnostics market share during the forecast period. Molecular diagnostic tests, specifically reverse transcriptase polymerase chain reaction (RT-PCR) tests, dominate the global market. RT-PCR tests are considered the ‘gold standard’ for COVID-19 diagnosis and are widely used due to high sensitivity and specificity. The test detects the presence of viral ribonucleic acid (RNA) in a patient's sample, such as a nasal or throat swab, and can confirm an active infection.
The RT-PCR tests segment held the second-largest share of the COVID-19 diagnostics market in 2021. This is ascribed to accurate results and increase in usage in testing centers and laboratories. These are the most preferred diagnostic tests for COVID-19 in several countries. The RT-PCR test is recommended as the most accurate diagnostic test for COVID-19, by the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC).
RT-PCR tests are relatively easy to perform and could be automated, which allows for a high throughput of samples to be processed. Therefore, these tests are suitable for large-scale testing in hospitals and laboratories.
Based on product, the reagents & kits segment held the largest global COVID-19 diagnostics industry share in 2021. These products include reagents and kits used in RT-PCR tests, rapid antigen tests, and serological tests (antibody tests). Reagents and kits are crucial components of diagnostic tests, as these are used to detect the presence of the SARS-CoV-2 virus in a patient's sample. The tests are used to extract the viral RNA from a patient's sample, amplify it, and detect the presence of the virus. Reagents and kits are also used to perform serological tests, which detect the presence of antibodies against the virus in a patient's blood sample.
Demand for diagnostic reagents and kits is high due to the need for accurate and reliable diagnostic tests for COVID-19. Thermo Fisher Scientific, QIAGEN, Roche, and Bio-Rad are major players in the diagnostic reagents & kits market.
In terms of end-user, the hospitals & clinics segment dominated the global COVID-19 diagnostics market in 2021. The COVID-19 pandemic has increased the demand for diagnostic tests, which has led to significant growth of the COVID-19 diagnostics market. Hospitals and clinics are among the primary providers of these tests. These settings have the necessary equipment, trained personnel, and infrastructure to conduct diagnostic tests, making them a crucial part of the COVID-19 testing ecosystem.
The at-home testing segment is anticipated to grow at a rapid pace during the forecast period. Demand for at-home COVID-19 testing has increased during the pandemic. The ability to test for the virus at home has several benefits, such as reduced risk of exposure to COVID-19 for individuals & healthcare workers, increase in access to testing for remote or underserved areas, and reduced burden on healthcare systems. Some companies have developed at-home testing kits that have been authorized by regulatory bodies, and are becoming widely available.
The U.S. is a major market for COVID-19 diagnostics in North America. The country has a large number of diagnostic centers & laboratories, and governments are investing significantly in COVID-19 diagnostic tests to help control the spread of the virus. The U.S. has one of the highest rates of COVID-19 testing in the world, which has led to significant increase in the demand for diagnostic tests. Additionally, the country has a large and well-established healthcare system, which has helped increase the adoption of diagnostic tests.
China, Japan, and South Korea are major markets for COVID-19 diagnostics in Asia Pacific. These countries have large number of diagnostic centers & laboratories. Governments have invested significantly in COVID-19 diagnostic tests to help control the spread of the virus. Asia Pacific has a large population and rapidly growing healthcare system, which has helped increase the adoption of diagnostic tests.
North America and Asia Pacific are two of the major markets for COVID-19 diagnostics, and their role in detecting and diagnosing COVID-19 cases is likely to continue to be important in the next few years.
Expansion of product portfolio and mergers & acquisitions are key strategies adopted by prominent manufacturers in the business. Abbott Laboratories, Becton, Dickinson and Company (BD), Bio-Rad Laboratories, Inc., Danaher Corporation (Cepheid), F. Hoffmann-La Roche Ltd. (Roche Diagnostics), Hologic, Inc., Laboratory Corporation of America Holdings, PerkinElmer, Inc., Quest Diagnostics, and Thermo Fisher Scientific, Inc. are key players in the industry.
The market report profiles key players based on parameters such as company overview, financial overview, strategies, portfolio, segments, and recent developments.
|
Attribute |
Detail |
|
Size in 2021 |
US$ 79.8 Bn |
|
Forecast (Value) in 2031 |
More than US$ 45.8 Bn |
|
Growth Rate (CAGR) |
-7.9% |
|
Forecast Period |
2022–2031 |
|
Historical Data Available for |
2019 & 2020 |
|
Quantitative Units |
US$ Mn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global market was valued at US$ 79.8 Bn in 2021
It is projected to reach more than US$ 45.8 Bn by 2031
The CAGR is anticipated to be -7.9% from 2022 to 2031
Increase in number of COVID-19 cases & testing and rise in demand for home diagnostic tests globally
North America is projected to account for significant market share during the forecast period
Abbott Laboratories, Becton, Dickinson and Company (BD), Bio-Rad Laboratories, Inc., Danaher Corporation (Cepheid), F. Hoffmann-La Roche Ltd (Roche Diagnostics), Hologic, Inc., Laboratory Corporation of America Holdings, PerkinElmer, Inc., Quest Diagnostics, and Thermo Fisher Scientific, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global COVID-19 Diagnostics Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global COVID-19 Diagnostics Market Analysis and Forecast, 2020–2031
5. Key Insights
5.1. Technological Advancements
5.2. COVID-19 Antigen Diagnostic Test Pipeline
5.3. Insights on FDA Approved COVID-19 Home Diagnostic Test
5.4. Regulatory Authorizations by Country for COVID-19 Diagnostic Tests
6. Global COVID-19 Diagnostics Market Analysis and Forecast, by Product
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Product, 2020–2031
6.3.1. Instruments
6.3.2. Reagents & Kits
6.4. Market Attractiveness Analysis, by Product
7. Global COVID-19 Diagnostics Market Analysis and Forecast, by Test Type
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Test Type, 2020–2031
7.3.1. Molecular Tests
7.3.2. Antigen Test
7.3.3. Antibody (serology) Tests
7.4. Market Attractiveness Analysis, by Test Type
8. Global COVID-19 Diagnostics Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by End-user, 2020–2031
8.3.1. Hospitals & Clinics
8.3.2. At-home Testing
8.3.3. Others
8.4. Market Attractiveness Analysis, by End-user
9. Global COVID-19 Diagnostics Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2020–2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America COVID-19 Diagnostics Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Product, 2020–2031
10.2.1. Instruments
10.2.2. Reagents & Kits
10.3. Market Value Forecast, by Test Type, 2020–2031
10.3.1. Molecular Tests
10.3.2. Antigen Test
10.3.3. Antibody (serology) Tests
10.4. Market Value Forecast, by End-user, 2020–2031
10.4.1. Hospitals & Clinics
10.4.2. At-home Testing
10.4.3. Others
10.5. Market Value Forecast, by Country, 2020–2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Product
10.6.2. By Test Type
10.6.3. By End-user
10.6.4. By Country
11. Europe COVID-19 Diagnostics Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Product, 2020–2031
11.2.1. Instruments
11.2.2. Reagents & Kits
11.3. Market Value Forecast, by Test Type, 2020–2031
11.3.1. Molecular Tests
11.3.2. Antigen Test
11.3.3. Antibody (serology) Tests
11.4. Market Value Forecast, by End-user, 2020–2031
11.4.1. Hospitals & Clinics
11.4.2. At-home Testing
11.4.3. Others
11.5. Market Value Forecast, by Country/Sub-region, 2020–2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Italy
11.5.5. Spain
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Product
11.6.2. By Test Type
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific COVID-19 Diagnostics Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Product, 2020–2031
12.2.1. Instruments
12.2.2. Reagents & Kits
12.3. Market Value Forecast, by Test Type, 2020–2031
12.3.1. Molecular Tests
12.3.2. Antigen Test
12.3.3. Antibody (Serology) Tests
12.4. Market Value Forecast, by End-user, 2020–2031
12.4.1. Hospitals & Clinics
12.4.2. At-home Testing
12.4.3. Others
12.5. Market Value Forecast, by Country/Sub-region, 2020–2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Product
12.6.2. By Test Type
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America COVID-19 Diagnostics Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Product, 2020–2031
13.2.1. Instruments
13.2.2. Reagents & Kits
13.3. Market Value Forecast, by Test Type, 2020–2031
13.3.1. Molecular Tests
13.3.2. Antigen Test
13.3.3. Antibody (serology) Tests
13.4. Market Value Forecast, by End-user, 2020–2031
13.4.1. Hospitals & Clinics
13.4.2. At-home Testing
13.4.3. Others
13.5. Market Value Forecast, by Country/Sub-region, 2020–2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Product
13.6.2. By Test Type
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa COVID-19 Diagnostics Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Product, 2020–2031
14.2.1. Instruments
14.2.2. Reagents & Kits
14.3. Market Value Forecast, by Test Type, 2020–2031
14.3.1. Molecular Tests
14.3.2. Antigen Test
14.3.3. Antibody (serology) Tests
14.4. Market Value Forecast, by End-user, 2020–2031
14.4.1. Hospitals & Clinics
14.4.2. At-home Testing
14.4.3. Others
14.5. Market Value Forecast, by Country/Sub-region, 2020–2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of MEA
14.6. Market Attractiveness Analysis
14.6.1. By Product
14.6.2. By Test Type
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competitive Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2021
15.3. Company Profiles
15.3.1. Abbott Laboratories
15.3.1.1. Company Overview
15.3.1.2. Product Portfolio
15.3.1.3. SWOT Analysis
15.3.1.4. Financial Overview
15.3.1.5. Strategic Overview
15.3.2. Becton, Dickinson and Company (BD)
15.3.2.1. Company Overview
15.3.2.2. Product Portfolio
15.3.2.3. SWOT Analysis
15.3.2.4. Financial Overview
15.3.2.5. Strategic Overview
15.3.3. Bio-Rad Laboratories, Inc.
15.3.3.1. Company Overview
15.3.3.2. Product Portfolio
15.3.3.3. SWOT Analysis
15.3.3.4. Financial Overview
15.3.3.5. Strategic Overview
15.3.4. Danaher Corporation (Cepheid)
15.3.4.1. Company Overview
15.3.4.2. Product Portfolio
15.3.4.3. SWOT Analysis
15.3.4.4. Financial Overview
15.3.4.5. Strategic Overview
15.3.5. F. Hoffmann-La Roche Ltd. (Roche Diagnostics)
15.3.5.1. Company Overview
15.3.5.2. Product Portfolio
15.3.5.3. SWOT Analysis
15.3.5.4. Financial Overview
15.3.5.5. Strategic Overview
15.3.6. Hologic, Inc.
15.3.6.1. Company Overview
15.3.6.2. Product Portfolio
15.3.6.3. SWOT Analysis
15.3.6.4. Financial Overview
15.3.6.5. Strategic Overview
15.3.7. Laboratory Corporation of America Holdings
15.3.7.1. Company Overview
15.3.7.2. Product Portfolio
15.3.7.3. SWOT Analysis
15.3.7.4. Financial Overview
15.3.7.5. Strategic Overview
15.3.8. PerkinElmer, Inc.
15.3.8.1. Company Overview
15.3.8.2. Product Portfolio
15.3.8.3. SWOT Analysis
15.3.8.4. Financial Overview
15.3.8.5. Strategic Overview
15.3.9. Quest Diagnostics
15.3.9.1. Company Overview
15.3.9.2. Product Portfolio
15.3.9.3. SWOT Analysis
15.3.9.4. Financial Overview
15.3.9.5. Strategic Overview
15.3.10. Thermo Fisher Scientific, Inc.
15.3.10.1. Company Overview
15.3.10.2. Product Portfolio
15.3.10.3. SWOT Analysis
15.3.10.4. Financial Overview
15.3.10.5. Strategic Overview
List of Tables
Table 01: Global COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 02: Global COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 03: Global COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
Table 04: Global COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Region, 2020–2031
Table 05: North America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Country, 2020–2031
Table 06: North America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 07: North America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 08: North America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
Table 09: Europe COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020–2031
Table 10: Europe COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 11: Europe COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 12: Europe COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
Table 13: Asia Pacific COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020–2031
Table 14: Asia Pacific COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 15: Asia Pacific COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 16: Asia Pacific COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
Table 17: Latin America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020–2031
Table 18: Latin America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 19: Latin America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 20: Latin America COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
Table 21: Middle East & Africa COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Country/Sub-region, 2020–2031
Table 22: Middle East & Africa COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Product, 2020–2031
Table 23: Middle East & Africa COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by Test Type, 2020–2031
Table 24: Middle East & Africa COVID-19 Diagnostics Market Size (US$ Mn) Forecast, by End-user, 2020–2031
List of Figures
Figure 01: Global COVID-19 Diagnostics Market Size (US$ Mn) and Distribution (%), by Region, 2021 and 2031
Figure 02: Global COVID-19 Diagnostics Market Revenue (US$ Mn), by Product, 2021
Figure 03: Global COVID-19 Diagnostics Market Value Share, by Product, 2021
Figure 04: Global COVID-19 Diagnostics Market Revenue (US$ Mn), by Test Type, 2021
Figure 05: Global COVID-19 Diagnostics Market Value Share, by Test Type, 2021
Figure 06: Global COVID-19 Diagnostics Market Revenue (US$ Mn), by End-user, 2021
Figure 07: Global COVID-19 Diagnostics Market Value Share, by End-user, 2021
Figure 08: Global COVID-19 Diagnostics Market Value Share, by Region, 2021
Figure 09: Global COVID-19 Diagnostics Market Value (US$ Mn) Forecast, 2020–2031
Figure 10: Global COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 11: Global COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022-2031
Figure 12: Global COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 13: Global COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022-2031
Figure 14: Global COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 15: Global COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022-2031
Figure 16: Global COVID-19 Diagnostics Market Value Share Analysis, by Region, 2021 and 2031
Figure 17: Global COVID-19 Diagnostics Market Attractiveness Analysis, by Region, 2022-2031
Figure 18: North America COVID-19 Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020–2031
Figure 19: North America COVID-19 Diagnostics Market Attractiveness Analysis, by Country, 2020–2031
Figure 20: North America COVID-19 Diagnostics Market Value Share Analysis, by Country, 2021 and 2031
Figure 21: North America COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 22: North America COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 23: North America COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 24: North America COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022–2031
Figure 25: North America COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 26:North America COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 27: Europe COVID-19 Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020–2031
Figure 28: Europe COVID-19 Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2020–2031
Figure 29: Europe COVID-19 Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 30: Europe COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 31: Europe COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 32: Europe COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 33: Europe COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022–2031
Figure 34: Europe COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 35: Europe COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 36: Asia Pacific COVID-19 Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020–2031
Figure 37: Asia Pacific COVID-19 Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2020–2031
Figure 38: Asia Pacific COVID-19 Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 39: Asia Pacific COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 40: Asia Pacific COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 41: Asia Pacific COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 42: Asia Pacific COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022–2031
Figure 43: Asia Pacific COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 44: Asia Pacific COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 45: Latin America COVID-19 Diagnostics Market Value (US$ Mn) Forecast and Y-o-Y Growth (%), 2020–2031
Figure 46: Latin America COVID-19 Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2020–2031
Figure 47: Latin America COVID-19 Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 48: Latin America COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 49: Latin America COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 50: Latin America COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 51: Latin America COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022–2031
Figure 52: Latin America COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 53: Latin America COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031
Figure 54: Middle East & Africa COVID-19 Diagnostics Market Size (US$ Mn) Forecast and Y-o-Y Growth (%), 2020–2031
Figure 55: Middle East & Africa COVID-19 Diagnostics Market Attractiveness Analysis, by Country/Sub-region, 2020–2031
Figure 56: Middle East & Africa COVID-19 Diagnostics Market Value Share Analysis, by Country/Sub-region, 2021 and 2031
Figure 57: Middle East & Africa COVID-19 Diagnostics Market Value Share Analysis, by Product, 2021 and 2031
Figure 58: Middle East & Africa COVID-19 Diagnostics Market Value Share Analysis, by Test Type, 2021 and 2031
Figure 59: Middle East & Africa COVID-19 Diagnostics Market Value Share Analysis, by End-user, 2021 and 2031
Figure 60: Middle East & Africa COVID-19 Diagnostics Market Attractiveness Analysis, by Product, 2022–2031
Figure 61: Middle East & Africa COVID-19 Diagnostics Market Attractiveness Analysis, by Test Type, 2022–2031
Figure 62: Middle East & Africa COVID-19 Diagnostics Market Attractiveness Analysis, by End-user, 2022–2031