The coronavirus pandemic has brought research labs and healthcare institutions under great scrutiny for accelerating the clinical trials for COVID-19 vaccines. As such, the success in these clinical trials has led to global recognition of India for supplying several lack of free doses to Brazil, Bangladesh, Algeria, and South Africa. Countries such as Sri Lanka are following suit whilst creating incremental opportunities for stakeholders in the clinical trials market.
The Food & Drug Administration (FDA) is creating awareness about Coronavirus Treatment Acceleration Program (CTAP) in order to bring economies to normal. Companies in the clinical trials market are taking advantage of this program to make new treatments available to patients as quickly as possible.

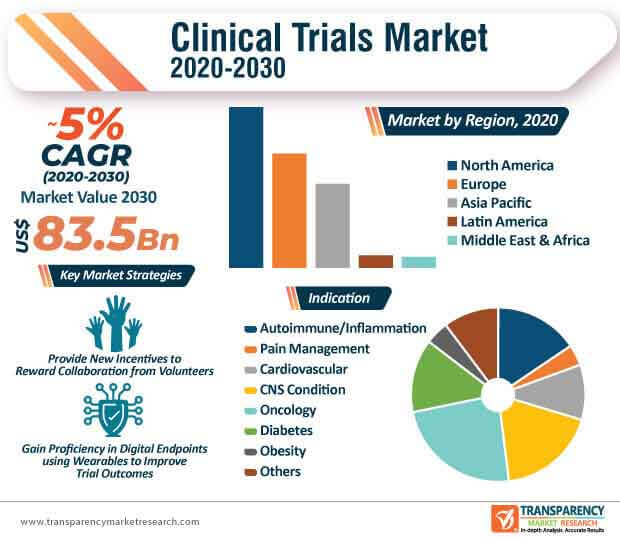
The clinical trials market is estimated to cross US$ 83.5 Bn by the end of 2030. However, finding the right patients is one of the most important pieces of the puzzle with respect to conducting clinical trials. Hence, stakeholders are working with experts at clinical trial recruitment companies to match patients with the right trials.
Slow recruitment is another challenge faced by companies in the market. Hence, companies are becoming aware about scrutinizing existing patient data to anticipate potential recruitment challenges in the long run. Clinical trial innovations are anticipated to bolster participation from volunteers.
Companies in the market are following guidelines of the FDA to advance in processes. They are referring to FDA guidance about Severely Debilitating or Life-Threatening Hematologic Disorders (SDLTHDs) in patients. Companies are increasing efforts to fill in the gap between scientific and technical complexities of clinical trials. Thus, companies are investing in improving the academia and are developing new incentives that reward collaboration from volunteers.
Companies in the market are taking additional efforts to develop networks that enable procedures at a patient’s home or at their private doctor’s clinic. This has led to innovations in sensor devices, patient reported outcomes on their computers, and flash pictures of their lesions from their cellphones that contribute toward processes.
Compliance with protocols is one of the key trends followed by stakeholders in the clinical trials market. Clinical trials supervised by Principal Investigators are gaining prominence in the market landscape. Continued positive cases for coronavirus have increased relevancy and role of stakeholders in the market. This shift has pushed clinical trials to go virtual.
Stakeholders in the market are teaming up with virtual clinical trial experts to capitalize on business opportunities during the ongoing pandemic. Even regulatory authorities have released guidelines to assist sponsors and patients via telemedicine and virtual trial tools to create viable solutions for current operational problems.
The proliferation of digital innovations with the help of wearables and sensors is translating into value grab opportunities for companies in the clinical trials market. ICON plc— a clinical research organization company is researching how digital endpoints, including digital biomarkers can improve trial outcomes. This has led to the adoption of digital health technologies that enable appropriate device selection and data strategies.
Digital health innovations hold promising potentials to better manage chronic diseases and improve patient access to healthcare services. Stakeholders and sponsors are taking efforts to improve adherence to medications and prevent its complications in patients. The Internet of Medical Things (IoMT) has the potential to enhance clinical development programs involving med-tech and pharmaceutical companies.
Analysts’ Viewpoint
There is a need for trial innovations since recently several volunteers were given wrong dose of the late stage clinical trial of the Oxford/AstraZeneca Covid-19 vaccine.
The clinical trials market is predicted to advance at a modest CAGR of 5.4% during the forecast period. This is evident since stakeholders need to address challenges pertaining to site management and compliance in order to improve trial outcomes. Hence, companies are developing clinical trial timelines to avoid delays, and are increasing transparency with their sponsors to offer them a 360 degree view of the processes happening at development centers. IoMT is acting as a key driver for the market.
Clinical Trials Market – Segmentation
| Phase |
|
| Study Design |
|
| Indication |
|
| Region |
|
It is projected to reach a value of US$ 83.5 Bn by the end of 2030
The CAGR is anticipated to be 5% from 2020 to 2030.
North America is expected to account for leading share during the forecast period.
High prevalence and increase in incidence rate of chronic diseases, and rise in R&D activities in biotechnology & pharmaceuticals industries are anticipated to drive the global market.
Laboratory Corporation of America Holdings, IQVIA, Inc., Syneos Health, Parexel International Corporation, PRA Health Sciences, Inc., PPD, Inc., Icon plc, Charles River Laboratories, Inc., WuXi AppTec, and Medpace Holdings, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Clinical Trials Market
4. Market Overview
4.1. Introduction
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Clinical Trials Market Analysis and Forecast, 2018–2030
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Number of Clinical Trials
5.2. Key Industry Events
5.3. COVID-19 Pandemic Impact on Industry
6. Global Clinical Trials Market Analysis and Forecast, by Phase
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Phase, 2018–2030
6.3.1. Phase I
6.3.2. Phase II
6.3.3. Phase III
6.3.4. Phase IV
6.4. Market Attractiveness Analysis, by Phase
7. Global Clinical Trials Market Analysis and Forecast, by Study Design
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Market Value Forecast, by Study Design, 2018–2030
7.3.1. Interventional Trials
7.3.2. Observational Trials
7.3.3. Expanded Access Trials
7.4. Market Attractiveness Analysis, by Study Design
8. Global Clinical Trials Market Analysis and Forecast, by Indication
8.1. Introduction & Definition
8.2. Key Findings / Developments
8.3. Market Value Forecast, by Indication, 2018–2030
8.3.1. Autoimmune/Inflammation
8.3.2. Pain Management
8.3.3. Cardiovascular
8.3.4. CNS Condition
8.3.5. Oncology
8.3.6. Diabetes
8.3.7. Obesity
8.3.8. Others
8.4. Market Attractiveness Analysis, by Indication
9. Global Clinical Trials Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Country/Region
10. North America Clinical Trials Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Phase, 2018–2030
10.2.1. Phase I
10.2.2. Phase II
10.2.3. Phase III
10.2.4. Phase IV
10.3. Market Value Forecast, by Study Design, 2018–2030
10.3.1. Interventional Trials
10.3.2. Observational Trials
10.3.3. Expanded Access Trials
10.4. Market Value Forecast, by Indication, 2018–2030
10.4.1. Autoimmune/Inflammation
10.4.2. Pain Management
10.4.3. Cardiovascular
10.4.4. CNS Condition
10.4.5. Oncology
10.4.6. Diabetes
10.4.7. Obesity
10.4.8. Others
10.5. Market Value Forecast, by Country, 2018–2030
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Phase
10.6.2. By Study Design
10.6.3. By Indication
10.6.4. By Country
11. Europe Clinical Trials Market Analysis and Forecast
11.1.Introduction
11.1.1. Key Findings
11.2.Market Value Forecast, by Phase, 2018–2030
11.2.1. Phase I
11.2.2. Phase II
11.2.3. Phase III
11.2.4. Phase IV
11.3.Market Value Forecast, by Study Design, 2018–2030
11.3.1. Interventional Trials
11.3.2. Observational Trials
11.3.3. Expanded Access Trials
11.4.Market Value Forecast, by Indication, 2018–2030
11.4.1. Autoimmune/Inflammation
11.4.2. Pain Management
11.4.3. Cardiovascular
11.4.4. CNS Condition
11.4.5. Oncology
11.4.6. Diabetes
11.4.7. Obesity
11.4.8. Others
11.5.Market Value Forecast, by Country/Sub-region, 2018–2030
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6.Market Attractiveness Analysis
11.6.1. By Phase
11.6.2. By Study Design
11.6.3. By Indication
11.6.4. By Country/Sub-region
12. Asia Pacific Clinical Trials Market Analysis and Forecast
12.1.Introduction
12.1.1. Key Findings
12.2.Market Value Forecast, by Phase, 2018–2030
12.2.1. Phase I
12.2.2. Phase II
12.2.3. Phase III
12.2.4. Phase IV
12.3.Market Value Forecast, by Study Design, 2018–2030
12.3.1. Interventional Trials
12.3.2. Observational Trials
12.3.3. Expanded Access Trials
12.4.Market Value Forecast, by Indication, 2018–2030
12.4.1. Autoimmune/Inflammation
12.4.2. Pain Management
12.4.3. Cardiovascular
12.4.4. CNS Condition
12.4.5. Oncology
12.4.6. Diabetes
12.4.7. Obesity
12.4.8. Others
12.5.Market Value Forecast, by Country/Sub-region, 2018–2030
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6.Market Attractiveness Analysis
12.6.1. By Phase
12.6.2. By Study Design
12.6.3. By Indication
12.6.4. By Country/Sub-region
13. Latin America Clinical Trials Market Analysis and Forecast
13.1.Introduction
13.1.1. Key Findings
13.2.Market Value Forecast, by Phase, 2018–2030
13.2.1. Phase I
13.2.2. Phase II
13.2.3. Phase III
13.2.4. Phase IV
13.3.Market Value Forecast, by Study Design, 2018–2030
13.3.1. Interventional Trials
13.3.2. Observational Trials
13.3.3. Expanded Access Trials
13.4.Market Value Forecast, by Indication, 2018–2030
13.4.1. Autoimmune/Inflammation
13.4.2. Pain Management
13.4.3. Cardiovascular
13.4.4. CNS Condition
13.4.5. Oncology
13.4.6. Diabetes
13.4.7. Obesity
13.4.8. Others
13.5.Market Value Forecast, by Country/Sub-region, 2018–2030
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6.Market Attractiveness Analysis
13.6.1. By Phase
13.6.2. By Study Design
13.6.3. By Indication
13.6.4. By Country/Sub-region
14. Middle East & Africa Clinical Trials Market Analysis and Forecast
14.1.Introduction
14.1.1. Key Findings
14.2.Market Value Forecast, by Phase, 2018–2030
14.2.1. Phase I
14.2.2. Phase II
14.2.3. Phase III
14.2.4. Phase IV
14.3.Market Value Forecast, by Study Design, 2018–2030
14.3.1. Interventional Trials
14.3.2. Observational Trials
14.3.3. Expanded Access Trials
14.4.Market Value Forecast, by Indication, 2018–2030
14.4.1. Autoimmune/Inflammation
14.4.2. Pain Management
14.4.3. Cardiovascular
14.4.4. CNS Condition
14.4.5. Oncology
14.4.6. Diabetes
14.4.7. Obesity
14.4.8. Others
14.5.Market Value Forecast, by Country/Sub-region, 2018–2030
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6.Market Attractiveness Analysis
14.6.1. By Phase
14.6.2. By Study Design
14.6.3. By Indication
14.6.4. By Country/Sub-region
15. Competitive Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2019
15.3. Company Profiles
15.3.1. Laboratory Corporation of America Holdings
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Financial Overview
15.3.1.3. Product Portfolio
15.3.1.4. Strategic Overview
15.3.1.5. SWOT Analysis
15.3.2. IQVIA Inc.
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Financial Overview
15.3.2.3. Product Portfolio
15.3.2.4. Strategic Overview
15.3.2.5. SWOT Analysis
15.3.3. Syneos Health
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Financial Overview
15.3.3.3. Product Portfolio
15.3.3.4. Strategic Overview
15.3.3.5. SWOT Analysis
15.3.4. Parexel International Corporation
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Financial Overview
15.3.4.3. Product Portfolio
15.3.4.4. Strategic Overview
15.3.4.5. SWOT Analysis
15.3.5. PPD Inc.
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Financial Overview
15.3.5.3. Product Portfolio
15.3.5.4. Strategic Overview
15.3.5.5. SWOT Analysis
15.3.6. Icon plc
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Financial Overview
15.3.6.3. Product Portfolio
15.3.6.4. Strategic Overview
15.3.6.5. SWOT Analysis
15.3.7. Charles River Laboratories, Inc.
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Financial Overview
15.3.7.3. Product Portfolio
15.3.7.4. Strategic Overview
15.3.3.5. SWOT Analysis
15.3.8. WuXi AppTec
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Financial Overview
15.3.8.3. Product Portfolio
15.3.8.4. Strategic Overview
15.3.8.5. SWOT Analysis
15.3.9. Medpace Holdings, Inc.
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Financial Overview
15.3.9.3. Product Portfolio
15.3.9.4. Strategic Overview
15.3.9.5. SWOT Analysis
List of Tables
Table 01: Global Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 02: Global Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 03: Global Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 04: Global Clinical Trials Market Value (US$ Mn) Forecast, by Region, 2018–2030
Table 05: North America Clinical Trials Market Value (US$ Mn) Forecast, by Country, 2018–2030
Table 06: North America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 07: North America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 08: North America Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 09: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 10: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 11: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 12: Europe Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 13: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 14: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 15: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 16: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 17: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 18: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 19: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 20: Latin America Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
Table 21: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Country/Sub-region, 2018–2030
Table 22: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Phase, 2018–2030
Table 23: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Study Design, 2018–2030
Table 24: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, by Indication, 2018–2030
List of Figures
Figure 01: Global Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 02: Global Clinical Trials Market Value Share, by Phase, 2019
Figure 03: Global Clinical Trials Market Value Share, by Indication, 2019
Figure 04: Global Clinical Trials Market Value Share, by Study Design, 2019
Figure 05: Global Clinical Trials Market Value Share, by Region, 2019
Figure 06: Global Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 07: Global Clinical Trials Market Value (US$ Mn), by Phase I, 2018–2030
Figure 08: Global Clinical Trials Market Value (US$ Mn), by Phase II, 2018–2030
Figure 09: Global Clinical Trials Market Value (US$ Mn), by Phase III, 2018–2030
Figure 10: Global Clinical Trials Market Value (US$ Mn), by Phase IV, 2018–2030
Figure 11: Global Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 12: Global Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 13: Global Clinical Trials Market Value (US$ Mn), Interventional Trials, 2018–2030
Figure 14: Global Clinical Trials Market Value (US$ Mn), Observational Trials, 2018–2030
Figure 15: Global Clinical Trials Market Value (US$ Mn), Expanded Access Trials, 2018–2030
Figure 16: Global Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 17: Global Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 18: Global Clinical Trials Market Value (US$ Mn), Autoimmune /Inflammation, 2018–2030
Figure 19: Global Clinical Trials Market Value (US$ Mn), Pain Management, 2018–2030
Figure 20: Global Clinical Trials Market Value (US$ Mn), Cardiovascular, 2018–2030
Figure 21: Global Clinical Trials Market Value (US$ Mn), CNS Condition, 2018–2030
Figure 22: Global Clinical Trials Market Value (US$ Mn), Oncology, 2018–2030
Figure 23: Global Clinical Trials Market Value (US$ Mn), Diabetes, 2018–2030
Figure 24: Global Clinical Trials Market Value (US$ Mn), Obesity, 2018–2030
Figure 25: Global Clinical Trials Market Value (US$ Mn), Others, 2018–2030
Figure 26: Global Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 27: Global Clinical Trials Market Value Share Analysis, by Region, 2019 and 2030
Figure 28: Global Clinical Trials Market Attractiveness Analysis, by Region, 2020–2030
Figure 29: North America Clinical Trials Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2018–2030
Figure 30: North America Clinical Trials Market Value Share (%), by Country, 2019 and 2030
Figure 31: North America Clinical Trials Market Attractiveness, by Country, 2020–2030
Figure 32: North America Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 33: North America Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 34: North America Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 35: North America Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 36: North America Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 37: North America Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 38: Europe Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 39: Europe Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 40: Europe Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 41: Europe Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 42: Europe Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 43: Europe Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 44: Europe Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 45: Europe Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 46: Europe Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 47: Asia Pacific Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 48: Asia Pacific Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 49: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 50: Asia Pacific Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 51: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 52: Asia Pacific Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 53: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 54: Asia Pacific Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 55: Asia Pacific Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 56: Latin America Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 57: Latin America Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 58: Latin America Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 59: Latin America Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 60: Latin America Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 61: Latin America Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 62: Latin America Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 63: Latin America Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 64: Latin America Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 65: Middle East & Africa Clinical Trials Market Value (US$ Mn) Forecast, 2018–2030
Figure 66: Middle East & Africa Clinical Trials Market Value Share Analysis, by Country/Sub-region, 2019 and 2030
Figure 67: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Country/Sub-region, 2020–2030
Figure 68: Middle East & Africa Clinical Trials Market Value Share Analysis, by Phase, 2019 and 2030
Figure 69: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Phase, 2020–2030
Figure 70: Middle East & Africa Clinical Trials Market Value Share Analysis, by Study Design, 2019 and 2030
Figure 71: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Study Design, 2020–2030
Figure 72: Middle East & Africa Clinical Trials Market Value Share Analysis, by Indication, 2019 and 2030
Figure 73: Middle East & Africa Clinical Trials Market Attractiveness Analysis, by Indication, 2020–2030
Figure 74: Global Clinical Trials Market Share Analysis, by Company, 2019