Analysts’ Viewpoint
Expansion of the pharmaceutical and biotechnology industries is driving the global cell and gene therapy manufacturing QC market. Rapid development and adoption of cell & gene therapies are propelling demand for quality control measures. Surge in need to ensure safety, efficacy, and consistency of these therapies is fueling market expansion. Furthermore, stringent regulatory requirements to meet regulatory compliance and approval are expected to bolster the global cell and gene therapy manufacturing QC market size.
Technological advancements in analytical methods and testing techniques offer lucrative opportunities to market players. Companies are focusing on increasing investment in research and development in order to develop safer and more efficient cell & gene therapies.
Cell and gene therapies hold immense potential in revolutionizing the treatment of various diseases, including genetic disorders, cancer, and autoimmune conditions. As these therapies advance from the research phase to commercialization, ensuring the safety, efficacy, and consistency of the manufactured products becomes crucial. This is driving demand for quality control measures.
The market is highly competitive, with several companies specializing in providing quality control services and technologies. These companies offer a range of solutions, such as analytical testing services, quality control software, and consulting services, to support cell and gene therapy manufacturers. Additionally, collaborations and partnerships between industry players, research institutions, and regulatory bodies contribute to the expansion of the market.
Demand for cell and gene therapies is fueled by factors that highlight their potential to revolutionize disease treatment. One key factor is the concept of personalized medicine, where therapies can be customized based on an individual's specific genetic makeup and disease characteristics. This approach holds great promise in delivering more targeted and effective treatments, particularly for complex and challenging conditions.
Cell and gene therapies have demonstrated remarkable clinical breakthroughs in previously incurable diseases. For instance, CAR-T cell therapies have shown unprecedented success in treating certain types of leukemia and lymphoma, with high response rates and long-lasting remissions.
These success stories have generated significant excitement among patients, healthcare providers, and investors, thereby driving the demand for further advancements and research in the field.
Surge in demand for cell and gene therapies is also influenced by the support and recognition from regulatory agencies and healthcare systems. Regulatory bodies such as the FDA and EMA have established expedited approval pathways and frameworks specifically designed to accommodate the unique characteristics of these therapies. This regulatory support has encouraged investment and development in the field, subsequently boosting demand for manufacturing and quality control services to ensure the safety and efficacy of these therapies.
Advancements in analytical technologies have played a crucial role in propelling global cell and gene therapy manufacturing QC market growth. These technological advancements have significantly enhanced the capacity to analyze, characterize, and guarantee the quality of cell and gene therapy products.
Development and implementation of cutting-edge analytical techniques, including next-generation sequencing (NGS), mass spectrometry, flow cytometry, and imaging technologies, have revolutionized quality control practices in the field of cell and gene therapies. These advanced technologies offer improved sensitivity, resolution, and throughput, enabling a more comprehensive and precise assessment of various critical parameters.
For instance, NGS enables comprehensive profiling of the genomic and transcriptomic characteristics of cells and vectors used in cell and gene therapies. It facilitates the detection of genetic modifications, identification of potential off-target effects, and evaluation of vector integrity and stability. The valuable information obtained through NGS aids in ensuring the consistency and quality of therapeutic products.
In terms of component, the global consumables segment accounted for the largest global cell and gene therapy manufacturing QC market share in 2022. Consumables play a critical role in the quality control process, as these are essential for sample collection, preparation, and analysis.
These consumables include various items such as reagents, test kits, assay plates, pipettes, and tubes. Consistent demand for consumables is ascribed to the need to perform routine quality control testing throughout the cell and gene therapy manufacturing process. As the number of cell and gene therapy manufacturing facilities and research institutions increases, so does the demand for consumables.
The consumables segment is experiencing continuous growth due to recurring nature of quality control testing. Quality control is not a one-time event, but an ongoing process that ensures the safety, efficacy, and consistency of cell and gene therapies.
Regular testing is required to monitor the quality and integrity of raw materials, intermediates, and final products. This necessitates the constant supply and utilization of consumables, making it a fundamental component of the quality control process.
The segment benefits from the wide range and diversity of consumable products available in the market. Different types of consumables are required for various quality control tests, ranging from basic laboratory supplies to specialized reagents and assay kits. Availability of a comprehensive portfolio of consumables tailored for cell and gene therapy quality control applications drives the segment.
Based on the analytical method, the potency testing segment dominated the global cell and gene therapy manufacturing QC industry in 2022. Potency testing is a critical parameter in assessing the effectiveness and therapeutic potential of cell and gene therapies. It measures the biological activity or functional characteristics of the therapy, providing crucial insights into its efficacy. Potency testing helps ensure that the therapy meets the desired standards and delivers the intended therapeutic effect, making it a vital aspect of quality control.
Regulatory agencies emphasize the importance of potency testing in the evaluation and approval of cell and gene therapies. Potency is often a primary endpoint in clinical trials and is closely monitored during the manufacturing process. Regulatory guidelines provide specific requirements for potency testing, necessitating its implementation to demonstrate the therapeutic value and consistency of the therapies. This regulatory focus on potency testing drives the demand for analytical methods and expertise in this particular segment.
Potency testing methods have also witnessed significant advancements and innovations in the past few years. Development of robust and reliable analytical techniques, such as bioassays, molecular assays, and functional assays, has enhanced the accuracy, sensitivity, and specificity of potency testing. These sophisticated methods enable a more precise and comprehensive evaluation of the therapeutic potency, leading to improved quality control assessment.
In terms of end-user, the pharmaceutical & biotechnology companies segment held the leading share of the global cell and gene therapy manufacturing QC market demand in 2022. These companies are at the forefront of developing and manufacturing cell and gene therapies. They invest significantly in research and development to bring innovative therapies to the market. Hence, they have a substantial demand for quality control measures to ensure the safety, efficacy, and consistency of their products. Quality control is an integral part of their manufacturing processes, and they prioritize compliance with regulatory guidelines and industry standards.
Pharmaceutical & biotechnology companies have the resources and infrastructure to establish in-house quality control laboratories or collaborate with specialized quality control service providers. They have the expertise and personnel to perform complex analytical testing and interpretation of results. This allows them to have better control over the quality control process and ensure timely and accurate assessment of their cell and gene therapy products.
As per cell and gene therapy manufacturing QC market trends, North America dominated the global industry in 2022. The region is home to a robust and well-established pharmaceutical and biotechnology industry. North America houses numerous leading companies involved in cell and gene therapy research, development, and manufacturing. These companies invest significantly in quality control measures to ensure the safety, efficacy, and compliance of their products, driving demand for quality control services and technologies.
North America has a strong regulatory framework that supports the development and commercialization of cell and gene therapies. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) have implemented clear guidelines and expedited approval pathways for these therapies.
According to cell and gene therapy manufacturing QC market research, Europe has a well-developed infrastructure for healthcare and life sciences, including state-of-the-art laboratories and research facilities. The region boasts advanced analytical technologies and expertise in quality control testing. This allows companies in Europe to perform comprehensive and accurate assessment of cell and gene therapy products, ensuring their quality and consistency.
As per cell and gene therapy manufacturing QC market analysis, Asia Pacific is home to a large population base, providing a significant market for cell and gene therapies. The region also faces a high burden of diseases that can potentially be treated with advanced therapies. Increase in demand for innovative treatment options has spurred the development and manufacturing of cell and gene therapies in the region. Quality control plays a crucial role in ensuring the safety, efficacy, and consistency of these therapies, making it a priority for companies operating in Asia Pacific.
The global cell and gene therapy manufacturing QC market is fragmented, with the presence of large number of players. Merger & acquisition and collaborations are the key strategies adopted by market players to increase market share.
bioMérieux SA, Bio-Rad Laboratories, Inc., Bio-Techne Corporation, QIAGEN, Charles River Laboratories International, Inc., Lonza Group AG, Merck KGaA, Intertek Group plc, Thermo Fisher Scientific, Inc., Eurofins Scientific S.E., and F. Hoffmann-La Roche Ltd. are the prominent players in the market.
Each of the prominent players has been profiled in the cell and gene therapy manufacturing QC market report scope based on parameters such as company overview, financial overview, strategies, portfolio, segments, and recent developments.
| Attribute | Detail |
|---|---|
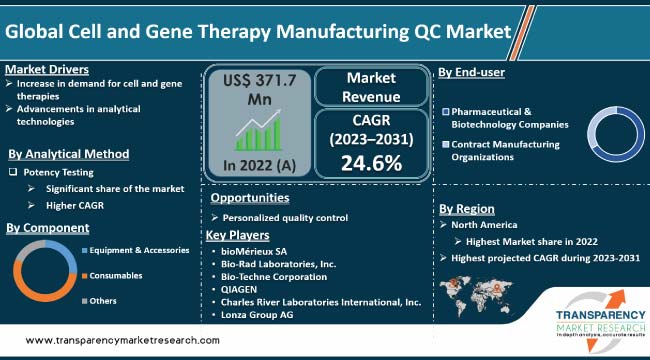
| Size in 2022 | US$ 371.7 Mn |
| Forecast Value in 2031 | More than US$ 2.8 Bn |
| Growth Rate (CAGR ) | 24.6% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Units | US$ Mn/Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Segmentation |
|
| Regions Covered |
|
| Countries Covered |
|
| Companies Profiled |
|
It was valued at US$ 371.7 Mn in 2022
It is projected to reach more than US$ 2.8 Bn by 2031
The CAGR is anticipated to be 24.6% from 2023 to 2031
North America is expected to account for the largest share from 2023 to 2031
bioMérieux SA, Bio-Rad Laboratories, Inc., Bio-Techne Corporation, QIAGEN, Charles River Laboratories International, Inc., Lonza Group AG, Merck KGaA, Intertek Group plc, Thermo Fisher Scientific, Inc., Eurofins Scientific S.E., and F. Hoffmann-La Roche Ltd.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Cell and Gene Therapy Manufacturing Quality Control (QC) Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast, 2017-2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Technological Advancements
5.2. Key Industry Events
5.3. Regulatory Scenario by Region/Globally
5.4. Major Research Institutes Involved
5.5. COVID-19 Pandemic Impact on Industry
6. Global Cell and Gene Therapy Manufacturing QC Analysis and Forecast, by Component
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Component, 2017-2031
6.3.1. Equipment & Accessories
6.3.2. Consumables
6.3.3. Others
6.4. Market Attractiveness Analysis, by Component
7. Global Cell and Gene Therapy Manufacturing QC Analysis and Forecast, by Analytical Method
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Analytical Method, 2017-2031
7.3.1. Sterility Testing
7.3.2. Purity Testing
7.3.3. Potency Testing
7.3.4. Identity Testing
7.3.5. Others
7.4. Market Attractiveness Analysis, by Analytical Method
8. Global Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast, by Process
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Process, 2017-2031
8.3.1. Upstream Processes
8.3.2. Downstream Processes
8.3.3. Process Development
8.4. Market Attractiveness Analysis, by Process
9. Global Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast, by End-user
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by End-user, 2017-2031
9.3.1. Pharmaceutical & Biotechnology Companies
9.3.2. Contract Manufacturing Organizations (CMOs)
9.4. Market Attractiveness Analysis, by End-user
10. Global Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast, by Region
10.1. Key Findings
10.2. Market Value Forecast, by Region, 2017-2031
10.2.1. North America
10.2.2. Europe
10.2.3. Asia Pacific
10.2.4. Latin America
10.2.5. Middle East & Africa
10.3. Market Attractiveness Analysis, by Region
11. North America Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Component, 2017-2031
11.2.1. Equipment & Accessories
11.2.2. Consumables
11.2.3. Others
11.3. Market Value Forecast, by Analytical Method, 2017-2031
11.3.1. Sterility Testing
11.3.2. Purity Testing
11.3.3. Potency Testing
11.3.4. Identity Testing
11.3.5. Others
11.4. Market Value Forecast, by Process, 2017-2031
11.4.1. Upstream Processes
11.4.2. Downstream Processes
11.4.3. Process Development
11.5. Market Value Forecast, by End-user, 2017-2031
11.5.1. Pharmaceutical & Biotechnology Companies
11.5.2. Contract Manufacturing Organizations (CMOs)
11.6. Market Value Forecast, by Country, 2017-2031
11.6.1. U.S.
11.6.2. Canada
11.7. Market Attractiveness Analysis
11.7.1. By Component
11.7.2. By Analytical Method
11.7.3. By Process
11.7.4. By End-user
11.7.5. By Country
12. Europe Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Component, 2017-2031
12.2.1. Equipment & Accessories
12.2.2. Consumables
12.2.3. Others
12.3. Market Value Forecast, by Analytical Method, 2017-2031
12.3.1. Sterility Testing
12.3.2. Purity Testing
12.3.3. Potency Testing
12.3.4. Identity Testing
12.3.5. Others
12.4. Market Value Forecast, by Process, 2017-2031
12.4.1. Upstream Processes
12.4.2. Downstream Processes
12.4.3. Process Development
12.5. Market Value Forecast, by End-user, 2017-2031
12.5.1. Pharmaceutical & Biotechnology Companies
12.5.2. Contract Manufacturing Organizations (CMOs)
12.6. Market Value Forecast, by Country/Sub-region, 2017-2031
12.6.1. Germany
12.6.2. U.K.
12.6.3. France
12.6.4. Italy
12.6.5. Spain
12.6.6. Rest of Europe
12.7. Market Attractiveness Analysis
12.7.1. By Component
12.7.2. By Analytical Method
12.7.3. By Process
12.7.4. By End-user
12.7.5. By Country/Sub-region
13. Asia Pacific Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Component, 2017-2031
13.2.1. Equipment & Accessories
13.2.2. Consumables
13.2.3. Others
13.3. Market Value Forecast, by Analytical Method, 2017-2031
13.3.1. Sterility Testing
13.3.2. Purity Testing
13.3.3. Potency Testing
13.3.4. Identity Testing
13.3.5. Others
13.4. Market Value Forecast, by Process, 2017-2031
13.4.1. Upstream Processes
13.4.2. Downstream Processes
13.4.3. Process Development
13.5. Market Value Forecast, by End-user, 2017-2031
13.5.1. Pharmaceutical & Biotechnology Companies
13.5.2. Contract Manufacturing Organizations (CMOs)
13.6. Market Value Forecast, by Country/Sub-region, 2017-2031
13.6.1. China
13.6.2. Japan
13.6.3. India
13.6.4. Australia & New Zealand
13.6.5. Rest of Asia Pacific
13.7. Market Attractiveness Analysis
13.7.1. By Component
13.7.2. By Analytical Method
13.7.3. By Process
13.7.4. By End-user
13.7.5. By Country/Sub-region
14. Latin America Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Component, 2017-2031
14.2.1. Equipment & Accessories
14.2.2. Consumables
14.2.3. Others
14.3. Market Value Forecast, by Analytical Method, 2017-2031
14.3.1. Sterility Testing
14.3.2. Purity Testing
14.3.3. Potency Testing
14.3.4. Identity Testing
14.3.5. Others
14.4. Market Value Forecast, by Process, 2017-2031
14.4.1. Upstream Processes
14.4.2. Downstream Processes
14.4.3. Process Development
14.5. Market Value Forecast, by End-user, 2017-2031
14.5.1. Pharmaceutical & Biotechnology Companies
14.5.2. Contract Manufacturing Organizations (CMOs)
14.6. Market Value Forecast, by Country/Sub-region, 2017-2031
14.6.1. Brazil
14.6.2. Mexico
14.6.3. Rest of Latin America
14.7. Market Attractiveness Analysis
14.7.1. By Component
14.7.2. By Analytical Method
14.7.3. By Process
14.7.4. By End-user
14.7.5. By Country/Sub-region
15. Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Component, 2017-2031
15.2.1. Equipment & Accessories
15.2.2. Consumables
15.2.3. Others
15.3. Market Value Forecast, by Analytical Method, 2017-2031
15.3.1. Sterility Testing
15.3.2. Purity Testing
15.3.3. Potency Testing
15.3.4. Identity Testing
15.3.5. Others
15.4. Market Value Forecast, by Process , 2017-2031
15.4.1. Upstream Processes
15.4.2. Downstream Processes
15.4.3. Process Development
15.5. Market Value Forecast, by End-user, 2017-2031
15.5.1. Pharmaceutical & Biotechnology Companies
15.5.2. Contract Manufacturing Organizations (CMOs)
15.6. Market Value Forecast, by Country/Sub-region, 2017-2031
15.6.1. GCC Countries
15.6.2. South Africa
15.6.3. Rest of Middle East & Africa
15.7. Market Attractiveness Analysis
15.7.1. By Component
15.7.2. By Analytical Method
15.7.3. By Process
15.7.4. By End-user
15.7.5. By Country/Sub-region
16. Competition Landscape
16.1. Market Player - Competition Matrix (by tier and size of companies)
16.2. Market Share Analysis, by Company, 2022
16.3. Company Profiles
16.3.1. bioMérieux SA
16.3.1.1. Company Overview
16.3.1.2. Financial Overview
16.3.1.3. Product Portfolio
16.3.1.4. Business Strategies
16.3.1.5. Recent Developments
16.3.2. Bio-Rad Laboratories, Inc.
16.3.2.1. Company Overview
16.3.2.2. Financial Overview
16.3.2.3. Product Portfolio
16.3.2.4. Business Strategies
16.3.2.5. Recent Developments
16.3.3. Bio-Techne Corporation
16.3.3.1. Company Overview
16.3.3.2. Financial Overview
16.3.3.3. Product Portfolio
16.3.3.4. Business Strategies
16.3.3.5. Recent Developments
16.3.4. QIAGEN
16.3.4.1. Company Overview
16.3.4.2. Financial Overview
16.3.4.3. Product Portfolio
16.3.4.4. Business Strategies
16.3.4.5. Recent Developments
16.3.5. Charles River Laboratories International, Inc.
16.3.5.1. Company Overview
16.3.5.2. Financial Overview
16.3.5.3. Product Portfolio
16.3.5.4. Business Strategies
16.3.5.5. Recent Developments
16.3.6. Lonza Group AG
16.3.6.1. Company Overview
16.3.6.2. Financial Overview
16.3.6.3. Product Portfolio
16.3.6.4. Business Strategies
16.3.6.5. Recent Developments
16.3.7. Merck KGaA
16.3.7.1. Company Overview
16.3.7.2. Financial Overview
16.3.7.3. Product Portfolio
16.3.7.4. Business Strategies
16.3.7.5. Recent Developments
16.3.8. Intertek Group plc
16.3.8.1. Company Overview
16.3.8.2. Financial Overview
16.3.8.3. Product Portfolio
16.3.8.4. Business Strategies
16.3.8.5. Recent Developments
16.3.9. Thermo Fisher Scientific Inc.
16.3.9.1. Company Overview
16.3.9.2. Financial Overview
16.3.9.3. Product Portfolio
16.3.9.4. Business Strategies
16.3.9.5. Recent Developments
16.3.10. Eurofins Scientific S.E.
16.3.10.1. Company Overview
16.3.10.2. Financial Overview
16.3.10.3. Product Portfolio
16.3.10.4. Business Strategies
16.3.10.5. Recent Developments
16.3.11. F. Hoffmann-La Roche Ltd.
16.3.11.1. Company Overview
16.3.11.2. Financial Overview
16.3.11.3. Product Portfolio
16.3.11.4. Business Strategies
16.3.11.5. Recent Developments
List of Tables
Table 01: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 02: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 03: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 04: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 05: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Region, 2017-2031
Table 06: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 07: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 08: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 09: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 10: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Country, 2017-2031
Table 11: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 12: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 13: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 14: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 15: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 16: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 17: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 18: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 19: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 20: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 21: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 22: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 23: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 24: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 25: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
Table 26: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 27: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Component, 2017-2031
Table 28: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Analytical Method, 2017-2031
Table 29: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by Process, 2017-2031
Table 30: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 02: Cell and Gene Therapy Manufacturing QC Market Value Share, by Component, 2022
Figure 03: Cell and Gene Therapy Manufacturing QC Market Value Share, by Product Type, 2022
Figure 04: Cell and Gene Therapy Manufacturing QC Market Value Share, by Process, 2022
Figure 05: Cell and Gene Therapy Manufacturing QC Market Value Share, by End-user, 2022
Figure 06: Global Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 07: Global Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component, 2023-2031
Figure 08: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn), by Equipment & Accessories, 2017‒2031
Figure 09: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn), by Consumables, 2017‒2031
Figure 10: Global Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn), by Others, 2017‒2031
Figure 11: Global Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 12: Global Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 13: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Sterility Testing, 2017-2031
Figure 14: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Urological Surgery, 2017-2031
Figure 15: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Potency Testing, 2017-2031
Figure 16: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Identity Testing, 2017-2031
Figure 17: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Others, 2017-2031
Figure 18: Global Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 19: Global Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Process, 2023-2031
Figure 20: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Upstream Processes, 2017-2031
Figure 21: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Downstream Processes, 2017-2031
Figure 22: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Process Development, 2017-2031
Figure 23: Global Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 24: Global Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031
Figure 25: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Pharmaceutical & Biotechnology Companies, 2017-2031
Figure 26: Global Cell and Gene Therapy Manufacturing QC Market Revenue (US$ Mn), by Contract Manufacturing Organizations (CMOs), 2017-31
Figure 27: Global Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Region, 2022 and 2031
Figure 28: Global Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Region, 2023-2031
Figure 29: North America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 30: North America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Country, 2022 and 2031
Figure 31: North America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Country, 2023-2031
Figure 32: North America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 33: North America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component 2023-2031
Figure 34: North America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 35: North America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 36: North America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 37: North America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, Process, 2023-2031
Figure 38: North America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 39: North America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031
Figure 40: Europe Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 41: Europe Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 42: Europe Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 43: Europe Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 44: Europe Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component 2023-2031
Figure 45: Europe Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 46: Europe Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 47: Europe Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 48: Europe Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, Process, 2023-2031
Figure 49: Europe Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 50: Europe Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031
Figure 51: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 52: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 53: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 54: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 55: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component 2023-2031
Figure 56: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 57: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 58: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 59: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, Process, 2023-2031
Figure 60: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 61: Asia Pacific Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031
Figure 62: Latin America Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 63: Latin America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 64: Latin America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 65: Latin America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 66: Latin America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component 2023-2031
Figure 67: Latin America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 68: Latin America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 69: Latin America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 70: Latin America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, Process, 2023-2031
Figure 71: Latin America Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 72: Latin America Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031
Figure 73: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value (US$ Mn) Forecast, 2017-2031
Figure 74: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 75: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 76: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Component, 2022 and 2031
Figure 77: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Component 2023-2031
Figure 78: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Analytical Method, 2022 and 2031
Figure 79: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by Analytical Method, 2023-2031
Figure 80: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by Process, 2022 and 2031
Figure 81: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, Process, 2023-2031
Figure 82: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Value Share Analysis, by End-user, 2022 and 2031
Figure 83: Middle East & Africa Cell and Gene Therapy Manufacturing QC Market Attractiveness Analysis, by End-user, 2023-2031