Demand for RNA-based COVID-19 Vaccines Creates Revenue Opportunities for Contact Manufacturers
Epidemiological studies indicate that patients with asthma are not at an increased risk of severe COVID-19. Although the severity of coronavirus in patients with asthma is dependent on many factors, currently, there is scarce information on the risks associated with COVID-19 in patients with severe asthma that consume biologics. Nevertheless, companies in the biologics contract manufacturing market are tapping revenue opportunities in vaccine production in order to reduce incidences of the novel infection.
Companies in the global biologics contract manufacturing market are shifting their focus to RNA-based COVID-19 vaccines. In order to meet the demands of the patients, an increasing number of pharmaceutical and biotech companies are entering into mergers & agreements with contract manufacturers to develop COVID-19 vaccines.
Process Intensification Holds Promising Potentials to Accelerate Business of Life-altering Therapeutics
In order to leverage value-grab opportunities in biologic therapies, it has become imperative to eliminate manufacturing bottlenecks with the help of process intensification (PI). Sartorius AG - an international pharmaceutical and laboratory equipment supplier, covering the segments of bioprocess solutions and lab products & services, is offering solutions for process intensification to accelerate the business of life-altering therapeutics during the ongoing pandemic.
Biologics complexities and long production cycles are fueling the need for PI. Such trends are contributing to the expansion of the biologics contract manufacturing market. PI is helping contract manufacturers to meet key regulatory deadlines, which is crucial for staying ahead in competition. This trend is essential in the manufacturing of biosimilars that need to be produced in several successive batches in a compressed timeline.
Commercialization of Ublituximab for Blood Cancer Treatment
The biologics contract manufacturing market is expected surpass US$ 47.3 Bn by 2031. Monoclonal antibodies are emerging as an important therapeutic approach for blood cancer treatment. TG Therapeutics, Inc. - a clinical-stage biopharmaceutical company, has announced to expand its contract manufacturing deal with Samsung Biologics - a South Korean biotechnology company, to support the production of its investigational anti-CD20 monoclonal antibody, ublituximab.
Companies in the biologics contract manufacturing market are entering into multiple collaborations to increase the availability of ublituximab and enable its commercialization. Companies are re-evaluating their supply needs and securing long-term capacity to meet the global demand for ublituximab.
Glass Containment Solutions and Drug Delivery Devices Ideal for Next-gen Biologics
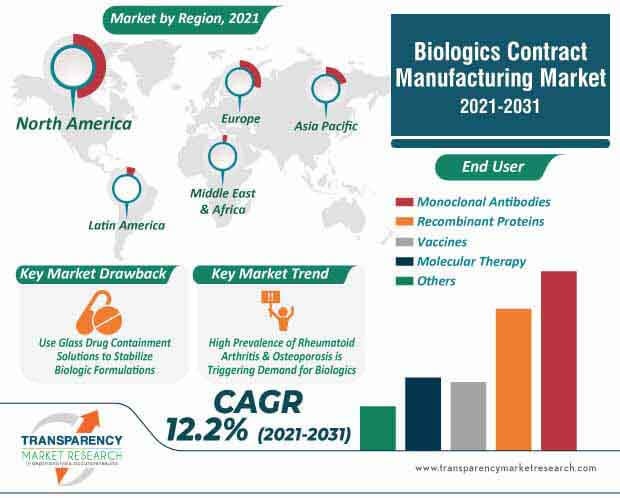
The biologics contract manufacturing market is projected to clock a CAGR of 12.2% during the forecast period. Stevanato Group - a specialist in world-class systems, processes, and services for pharmaceutical & healthcare industry, is providing novel glass drug containment solutions and drug delivery devices ideal for next-gen drugs.
With the help of state-of-the-art analytical services, reliable technology, and manufacturing equipment, companies in the biologics contract manufacturing market are able to capitalize on incremental opportunities. Biotech drugs such as recombinant proteins and monoclonal antibodies are fueling the demand for glass drug containment solutions and drug delivery devices. The high prevalence of rheumatoid arthritis and osteoporosis is triggering the demand for biologics.

Analysts’ Viewpoint
Due to very low margin for error in the production of life-altering therapeutics for COVID-19 treatment and prevention, companies in the biologics contract manufacturing market should adopt process intensification to avoid production failure. Companies are setting their collaboration wheels in motion to enable long-standing R&D activities involving monoclonal antibodies for blood cancer treatment. Since biologics are challenging to stabilize and administrate, especially in a syringe or a drug delivery device owing to their viscosity, complexity, and sensitivity, companies are using glass drug containment solutions to commercialize biologics. These solutions are necessary since biologics undergo stringent regulatory assessments as compared to generic drugs.
Biologics Contract Manufacturing Market: Overview
Growth of Biopharmaceutical Industry and Promising Drug Pipeline: Key Drivers
Increase in Investment in Research & Development
High Cost of Treatment to Restrain Biologics Contract Manufacturing Market
Biologics Contract Manufacturing Market: Competition Landscape
Biologics Contract Manufacturing Market: Key Developments
Biologics Contract Manufacturing Market Snapshot
|
Attribute |
Detail |
|
Market Size Value in 2020 (Base Year) |
US$ 13.3 Bn |
|
Market Forecast Value in 2031 |
US$ 47.3 Bn |
|
Growth Rate (CAGR) |
12.2% |
|
Forecast Period |
2021–2031 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes cross segment analysis at regional level. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, supply chain analysis, and parent industry overview. |
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
Biologics contract manufacturing market is expected surpass US$ 47.3 Bn by 2031
Biologics contract manufacturing market is projected to expand at a CAGR of 12.2% from 2021 to 2031
Biologics contract manufacturing market is driven by increase in investment in research & development activities and rise in prevalence of chronic diseases.
North America accounted for a major share of the global biologics contract manufacturing market
Key players operating in the global market include Lonza Group, Samsung Biologics Co., Ltd., Patheon by Thermo Fisher Scientific, Inc., Cambrex Corporation
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Biologics Contract Manufacturing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Biologics Contract Manufacturing Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Biologics Contract Manufacturing: Overview
5.2. Trends in Biopharma Contract Manufacturing
5.3. Key Industry Events (mergers, acquisitions, collaborations, approvals, etc.)
5.4. COVID-19 Pandemic Impact on Industry
6. Global Biologics Contract Manufacturing Market Analysis and Forecast, by Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value & Volume Forecast, by Type, 2017–2031
6.3.1. Monoclonal Antibodies
6.3.2. Recombinant Proteins
6.3.3. Vaccines
6.3.4. Molecular Therapy
6.3.5. Others
6.4. Market Attractiveness Analysis, by Type
7. Global Biologics Contract Manufacturing Market Analysis and Forecast, by Region
7.1. Key Findings
7.2. Market Value & Volume Forecast, by Region
7.2.1. North America
7.2.2. Europe
7.2.3. Asia Pacific
7.2.4. Latin America
7.2.5. Middle East & Africa
7.3. Market Attractiveness Analysis, by Country/Region
8. North America Biologics Contract Manufacturing Market Analysis and Forecast
8.1. Introduction
8.1.1. Key Findings
8.2. Market Value & Volume Forecast, by Type, 2017–2031
8.2.1. Monoclonal Antibodies
8.2.2. Recombinant Proteins
8.2.3. Vaccines
8.2.4. Molecular Therapy
8.2.5. Others
8.3. Market Value & Volume Forecast, by Country, 2017–2031
8.3.1. U.S.
8.3.2. Canada
8.4. Market Attractiveness Analysis
8.4.1. By Type
8.4.2. By Country
9. Europe Biologics Contract Manufacturing Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value & Volume Forecast, by Type, 2017–2031
9.2.1. Monoclonal Antibodies
9.2.2. Recombinant Proteins
9.2.3. Vaccines
9.2.4. Molecular Therapy
9.2.5. Others
9.3. Market Value & Volume Forecast, by Country/Sub-region, 2017–2031
9.3.1. Germany
9.3.2. U.K.
9.3.3. France
9.3.4. Spain
9.3.5. Italy
9.3.6. Rest of Europe
9.4. Market Attractiveness Analysis
9.4.1. By Type
9.4.2. By Country/Sub-region
10. Asia Pacific Biologics Contract Manufacturing Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value & Volume Forecast, by Type, 2017–2031
10.2.1. Monoclonal Antibodies
10.2.2. Recombinant Proteins
10.2.3. Vaccines
10.2.4. Molecular Therapy
10.2.5. Others
10.3. Market Value & Volume Forecast, by Country/Sub-region, 2017–2031
10.3.1. China
10.3.2. Japan
10.3.3. India
10.3.4. Australia & New Zealand
10.3.5. Rest of Asia Pacific
10.4. Market Attractiveness Analysis
10.4.1. By Type
10.4.2. By Country/Sub-region
11. Latin America Biologics Contract Manufacturing Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value & Volume Forecast, by Type, 2017–2031
11.2.1. Monoclonal Antibodies
11.2.2. Recombinant Proteins
11.2.3. Vaccines
11.2.4. Molecular Therapy
11.2.5. Others
11.3. Market Value & Volume Forecast, by Country//Sub-region, 2017–2031
11.3.1. Brazil
11.3.2. Mexico
11.3.3. Rest of Latin America
11.4. Market Attractiveness Analysis
11.4.1. By Type
11.4.2. By Country/Sub-region
12. Middle East & Africa Biologics Contract Manufacturing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value & Volume Forecast, by Type, 2017–2031
12.2.1. Monoclonal Antibodies
12.2.2. Recombinant Proteins
12.2.3. Vaccines
12.2.4. Molecular Therapy
12.2.5. Others
12.3. Market Value & Volume Forecast, by Country/Sub-region, 2017–2031
12.3.1. GCC Countries
12.3.2. South Africa
12.3.3. Rest of Middle East & Africa
12.4. Market Attractiveness Analysis
12.4.1. By Type
12.4.2. By Country/Sub-region
13. Competition Landscape
13.1. Company Profiles
13.1.1. Lonza Group
13.1.1.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.1.2. Product Portfolio
13.1.1.3. SWOT Analysis
13.1.1.4. Strategic Overview
13.1.2. Samsung Biologics Co., Ltd.
13.1.2.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.2.2. Product Portfolio
13.1.2.3. SWOT Analysis
13.1.2.4. Strategic Overview
13.1.3. Patheon by Thermo Fisher Scientific, Inc.
13.1.3.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.3.2. Product Portfolio
13.1.3.2. SWOT Analysis
13.1.3.3. Strategic Overview
13.1.4. Cambrex Corporation
13.1.4.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.4.2. Product Portfolio
13.1.4.3. SWOT Analysis
13.1.4.4. Strategic Overview
13.1.5. Siegfried Holding AG
13.1.5.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.5.2. Product Portfolio
13.1.5.3. SWOT Analysis
13.1.5.4. Strategic Overview
13.1.6. Fujifilm Holding Corporation
13.1.6.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.6.2. Product Portfolio
13.1.6.3. SWOT Analysis
13.1.6.4. Strategic Overview
13.1.7. AbbVie, Inc.
13.1.7.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.7.2. Product Portfolio
13.1.7.3. SWOT Analysis
13.1.7.4. Strategic Overview
13.1.8. Boehringer Ingelheim
13.1.8.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.8.2. Product Portfolio
13.1.8.3. SWOT Analysis
13.1.8.4. Strategic Overview
13.1.9. Recipharm Pharmaceuticals
13.1.9.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.9.2. Product Portfolio
13.1.9.3. SWOT Analysis
13.1.9.4. Strategic Overview
13.1.10. WuXi Biologics
13.1.10.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.10.2. Product Portfolio
13.1.10.3. SWOT Analysis
13.1.10.4. Strategic Overview
13.1.11. Catalent, Inc.
13.1.11.1. Company Overview (HQ, Business Segments, Employee Strength)
13.1.11.2. Product Portfolio
13.1.11.3. SWOT Analysis
13.1.11.4. Strategic Overview
List of Tables
Table 01: Global Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017‒2031
Table 02: Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 03: North America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country, 2017–2031
Table 04: North America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 05: Europe Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-Region, 2017–2031
Table 06: Europe Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 07: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 08: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 09: Latin America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017-2031
Table 10: Latin America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 11: Middle East & Africa Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-Region, 2017–2031
Table 12: Middle East & Africa Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
List of Figures
Figure 01: Global Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Biologics Contract Manufacturing Market Value Share, by Type, 2020
Figure 03: Biologics Contract Manufacturing Market Value Share, by Region, 2020
Figure 04: Global Biologics Contract Manufacturing Market Value Share Analysis, by Type, 2020 and 2031
Figure 05: Global Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 06: Global Biologics Contract Manufacturing Market Value (US$ Mn), by Monoclonal Antibodies, 2017‒2031
Figure 07: Global Biologics Contract Manufacturing Market Value (US$ Mn), by Recombinant Proteins, 2017‒2031
Figure 08: Global Biologics Contract Manufacturing Market Value (US$ Mn), by Vaccines, 2017‒2031
Figure 09: Global Biologics Contract Manufacturing Market Value (US$ Mn), by Molecular Therapy, 2017‒2031
Figure 10: Global Biologics Contract Manufacturing Market Value (US$ Mn), by Others, 2017–2031
Figure 11: Global Biologics Contract Manufacturing Market Value Share Analysis, by Region, 2020 and 2031
Figure 12: Global Biologics Contract Manufacturing Market Attractiveness Analysis, by Region, 2021–2031
Figure 13: North America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 14: North America Biologics Contract Manufacturing Market Value Share Analysis, by Country, 2020 and 2031
Figure 15: North America Biologics Contract Manufacturing Market Attractiveness Analysis, by Country, 2021–2031
Figure 16: North America Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 17: North America Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 18: Europe Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 19: Europe Biologics Contract Manufacturing Market Value Share Analysis, by Country/Sub-Region, 2020 and 2031
Figure 20: Europe Biologics Contract Manufacturing Market Attractiveness Analysis, by Country/Sub-region, 2021–2031
Figure 21: Europe Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 22: Europe Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 23: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 24: Asia Pacific Biologics Contract Manufacturing Market Value Share Analysis, by Country/Sub-Region, 2020 and 2031
Figure 25: Asia Pacific Biologics Contract Manufacturing Market Attractiveness Analysis, by Country/Sub-Region, 2021-2031
Figure 26: Asia Pacific Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 27: Asia Pacific Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 28: Latin America Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 29: Latin America Biologics Contract Manufacturing Market Value Share Analysis, by Country/Sub-Region, 2020 and 2031
Figure 30: Latin America Biologics Contract Manufacturing Market Attractiveness Analysis, by Country/Sub-Region, 2021-2031
Figure 31: Latin America Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 32: Latin America Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 33: Middle East & Africa Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 34: Middle East & Africa Biologics Contract Manufacturing Market Value Share Analysis, by Country/Sub-Region, 2020 and 2031
Figure 35: Middle East & Africa Biologics Contract Manufacturing Market Attractiveness Analysis, by Country/Sub-Region, 2021–2031
Figure 36: Middle East & Africa Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 37: Middle East & Africa Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031