Since hormones, fusion proteins, and vaccines form an important part of biologics, bioreactor sales are witnessing an upsurge during the COVID-19 outbreak. Such trends are contributing to the growth of the Asia Pacific biologics contract manufacturing market. Bioreactor sales have registered a notable increase during 2020 with many companies expanding their facilities in order to fulfil the demand for COVID-19 vaccine production.
Nationwide vaccination programs are creating revenue opportunities for stakeholders in the Asia Pacific biologics contract manufacturing market. The increasing government focus on encouraging the growth of local biotech companies by offering direct investments, tax incentives, and other resources is growing prominent in countries of Asia Pacific. Since vaccination is an underutilized public health intervention, stakeholders are working closely with governments to deploy initiatives for immunization of populations.
The patient-specific therapy is gaining importance for biopharmaceutical research. Biologics manufacturing challenges can emerge in multiple areas, but leading bio-manufacturers are addressing these difficulties with the help of process intensification (PI). Process intensification refers to a list of comprehensive initiatives that can maximize the efficiency of a bioprocess and make a biologic product more successful.
The perennial problem of productivity is affecting the growth of the Asia Pacific biologics contract manufacturing market. Thus, process intensification holds promising potentials to help introduce new products in a shorter period of time. With greater production capacity, biopharma organizations can scale their processes to meet patient demands.
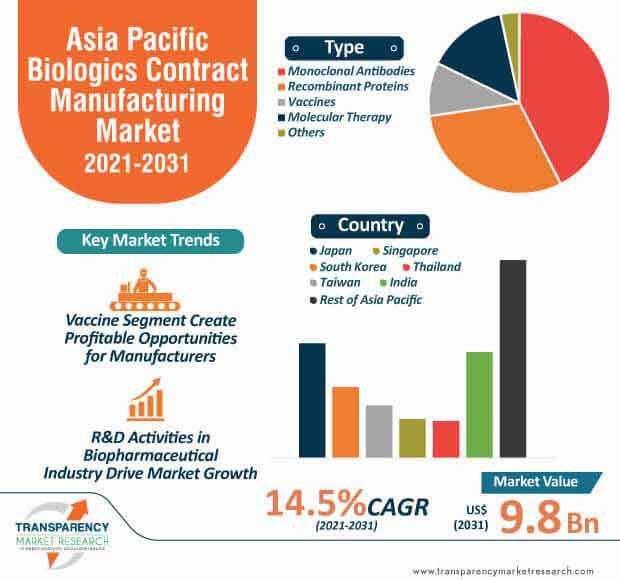
The Asia Pacific biologics contract manufacturing market is expected to register a CAGR of 14.5% during the forecast period. In order to overcome challenges in biologics contract manufacturing, optimized digital manufacturing is gaining prominence as an essential remedy to boost productivity levels and adhere to high compliance standards. This strategy is helping biopharma organizations to become more technologically progressive and adaptive than other manufacturers.
Optimized digital manufacturing is helping companies in the Asia Pacific biologics contract manufacturing market to accelerate product innovations and ramp-up projects as per market demand trends. The automation of manufacturing processes is another key driver of the market.
The Asia Pacific biologics contract manufacturing market is projected to reach the valuation of US$ 9.8 Bn by 2031. The high prevalence of cancer is catalyzing the demand for biologics. However, the steep cost of these drugs is acting as a barrier to patient access. Nevertheless, patents in several oncology biologics are predicted to expire during the upcoming years, which is creating an opportunity for companies to introduce cost-efficient biosimilars.
Pharmaceutical manufacturers in the Asia Pacific biologics contract manufacturing market are shifting their focus from generics and joining the fray motivated by advantageous global and local regulations. For instance, Celltrion - a biopharmaceutical company headquartered in Incheon, South Korea, is acquiring recognition for successfully gaining regulatory approval in Europe companies with infliximab, a monoclonal antibody.
Even though patients may find the price of biologics to be high, Asia-based companies are at an advantageous position, since a lower cost base relative to countries in Europe and the U.S. is grabbing attention of stakeholders. Companies in the Asia Pacific biologics contract manufacturing market are capitalizing on low capital expenditure for manufacturing facilities, low biosimilar development costs, and cost efficient labor to advance in the healthcare landscape.
In China, a regulatory change permitting pharmaceutical companies to outsource manufacturing to CMOs (Contract Manufacturing Organizations) has led to new opportunities for biotechs to concentrate on innovative drug development. There is a strong intent of companies located in Asia to bring biosilimars into western markets.
Analysts’ Viewpoint
Companies in the Asia Pacific biologics contract manufacturing market are upgrading their infrastructure to accelerate the process development of COVID-19 vaccines. With the help of process intensification, stakeholders are deploying services that boost production whilst reducing the carbon footprint. Although biosimilars offer a similar clinical outcome as their reference counterparts at a reduced price, it is still important for manufacturers to seek recognition in the U.S., which limits its uptake. Nevertheless, biopharma organizations in Asia Pacific should stay flexible and respond to changing demands of patients. Research breakthroughs and new molecule discoveries are contributing to market expansion. The process intensification can help bio-manufacturing efforts become more responsive as technologies evolve rapidly.
Asia Pacific Biologics Contract Manufacturing Market Snapshot
|
Attribute |
Detail |
|
Market Size Value in 2020 (Base Year) |
US$ 2.4 Bn |
|
Market Forecast Value in 2031 |
US$ 9.8 Bn |
|
Growth Rate (CAGR) |
14.5% |
|
Forecast Period |
2021–2031 |
|
Quantitative Units |
US$ Mn for Value |
|
Market Analysis |
It includes cross segment analysis at regional level. Furthermore, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, supply chain analysis, and parent industry overview. |
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Covered |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
Biologics contract manufacturing market in asia pacific is projected to reach the valuation of US$ 9.8 Bn by 2031
Biologics contract manufacturing market in asia pacific to expand at a CAGR of 14.5% from 2021 to 2031
Biologics contract manufacturing market is driven by increase in sales of biopharmaceuticals and number of molecules in the pipeline being developed by small and virtual biotech companies
The monoclonal antibodies segment dominated the asia pacific biologics contract manufacturing marketand the trend is projected to continue during the forecast period
Key players in asia pacific biologics contract manufacturing markets include Lonza Group, Samsung Biologics Co., Ltd., Patheon by Thermo Fisher Scientific, Inc., Cambrex Corporation, Siegfried Holding AG, Others
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global & Asia Pacific Biologics Contract Manufacturing Market
4. Market Overview
4.1. Introduction
4.1.1. Segment Definition
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global & Asia Pacific Biologics Contract Manufacturing Market Analysis and Forecast, 2017–2031
4.4.1. Market Revenue Projections (US$ Mn)
5. Key Insights
5.1. Biologics Contract Manufacturing: Overview
5.2. Trends in Biopharma Contract Manufacturing
5.3. Key Industry Events (mergers, acquisitions, collaborations, approvals, etc.)
5.4. COVID-19 Pandemic Impact on Industry
6. Global & Asia Pacific Biologics Contract Manufacturing Market Analysis and Forecast, by Type
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Market Value Forecast, by Type, 2017–2031
6.3.1. Monoclonal Antibodies
6.3.2. Recombinant Proteins
6.3.3. Vaccines
6.3.4. Molecular Therapy
6.3.5. Others
6.4. Market Attractiveness Analysis, by Type
7. Asia Pacific Biologics Contract Manufacturing Market Analysis and Forecast, by Country/Sub-Region
7.1. Key Findings
7.2. Market Value Forecast, by Country/Sub-Region
7.2.1. Japan
7.2.2. South Korea
7.2.3. Taiwan
7.2.4. Singapore
7.2.5. Thailand
7.2.6. India
7.2.7. Rest of Asia Pacific
7.3. Market Attractiveness Analysis, by Country/Sub-Region
8. Japan Biologics Contract Manufacturing Market Analysis and Forecast
8.1. Introduction
8.1.1. Key Findings
8.2. Market Value Forecast, by Type, 2017–2031
8.2.1. Monoclonal Antibodies
8.2.2. Recombinant Proteins
8.2.3. Vaccines
8.2.4. Molecular Therapy
8.2.5. Others
8.3. Market Attractiveness Analysis
8.3.1. By Type
9. South Korea Biologics Contract Manufacturing Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.2. Market Value Forecast, by Type, 2017–2031
9.2.1. Monoclonal Antibodies
9.2.2. Recombinant Proteins
9.2.3. Vaccines
9.2.4. Molecular Therapy
9.2.5. Others
9.3. Market Attractiveness Analysis
9.3.1. By Type
10. Taiwan Biologics Contract Manufacturing Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Type, 2017–2031
10.2.1. Monoclonal Antibodies
10.2.2. Recombinant Proteins
10.2.3. Vaccines
10.2.4. Molecular Therapy
10.2.5. Others
10.3. Market Attractiveness Analysis
10.3.1. By Type
11. Singapore Biologics Contract Manufacturing Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Type, 2017–2031
11.2.1. Monoclonal Antibodies
11.2.2. Recombinant Proteins
11.2.3. Vaccines
11.2.4. Molecular Therapy
11.2.5. Others
11.3. Market Attractiveness Analysis
11.3.1. By Type
12. Thailand Biologics Contract Manufacturing Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Type, 2017–2031
12.2.1. Monoclonal Antibodies
12.2.2. Recombinant Proteins
12.2.3. Vaccines
12.2.4. Molecular Therapy
12.2.5. Others
12.3. Market Attractiveness Analysis
12.3.1. By Type
13. India Biologics Contract Manufacturing Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Type, 2017–2031
13.2.1. Monoclonal Antibodies
13.2.2. Recombinant Proteins
13.2.3. Vaccines
13.2.4. Molecular Therapy
13.2.5. Others
13.3. Market Attractiveness Analysis
13.3.1. By Type
14. Rest of Asia Pacific Biologics Contract Manufacturing Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Type, 2017–2031
14.2.1. Monoclonal Antibodies
14.2.2. Recombinant Proteins
14.2.3. Vaccines
14.2.4. Molecular Therapy
14.2.5. Others
14.3. Market Attractiveness Analysis
14.3.1. By Type
15. Competition Landscape
15.1. Market Player - Competition Matrix (by tier and size of companies)
15.2. Market Share Analysis, by Company, 2020
15.3. Company Profiles
15.3.1. Lonza Group
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Strategic Overview
15.3.1.4. SWOT Analysis
15.3.2. Samsung Biologics Co., Ltd.
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Strategic Overview
15.3.2.4. SWOT Analysis
15.3.3. Patheon by Thermo Fisher Scientific, Inc.
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Strategic Overview
15.3.3.4. SWOT Analysis
15.3.4. Siegfried Holding AG
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Strategic Overview
15.3.4.4. SWOT Analysis
15.3.5. Cambrex Corporation
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Strategic Overview
15.3.5.4. SWOT Analysis
15.3.6. Boehringer Ingelheim
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Strategic Overview
15.3.6.4. SWOT Analysis
15.3.7. Recipharm Pharmaceuticals
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Strategic Overview
15.3.7.4. SWOT Analysis
15.3.8. Catalent Inc.
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Strategic Overview
15.3.8.4. SWOT Analysis
15.3.9. WuXi Biologics
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Strategic Overview
15.3.9.4. SWOT Analysis
15.3.10. Fujifilm Holding Corporation
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. Strategic Overview
15.3.10.4. SWOT Analysis
15.3.11. AbbVie, Inc.
15.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.11.2. Product Portfolio
15.3.11.3. Strategic Overview
15.3.11.4. SWOT Analysis
List of Tables
Table 01: Global Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017‒2031
Table 02: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Country/Sub-Region, 2017–2031
Table 03: Japan Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 04: South Korea Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 05: Taiwan Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 06: Singapore Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 07: Thailand Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 08: India Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 09: Rest of Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, by Type, 2017–2031
List of Figures
Figure 01: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Asia Pacific Biologics Contract Manufacturing Market Value Share, by Type, 2020
Figure 03: Asia Pacific Biologics Contract Manufacturing Market Value Share, by Country/Sub-Regions, 2020
Figure 04: Asia Pacific Biologics Contract Manufacturing Market Value Share Analysis, by Type, 2020 and 2031
Figure 05: Asia Pacific Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 06: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn), by Monoclonal Antibodies, 2017‒2031
Figure 07: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn), by Recombinant Proteins, 2017‒2031
Figure 08: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn), by Vaccines, 2017‒2031
Figure 09: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn), by Molecular Therapy, 2017‒2031
Figure 10: Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn), by Others, 2017–2031
Figure 11: Asia Pacific Biologics Contract Manufacturing Market Attractiveness Analysis, by Region, 2021–2031
Figure 12: Asia Pacific Biologics Contract Manufacturing Market Value Share Analysis, by Region, 2020 and 2031
Figure 13: Japan Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 14: Japan Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 15: Japan Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 16: South Korea Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 17: South Korea Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 18: South Korea Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 19: Taiwan Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 20: Taiwan Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 21: Taiwan Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 22: Singapore Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 23: Singapore Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 24: Singapore Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 25: Thailand Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 26: Thailand Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 27: Thailand Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 28: India Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 29: India Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 30: India Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031
Figure 31: Rest of Asia Pacific Biologics Contract Manufacturing Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 32: Rest of Asia Pacific Biologics Contract Manufacturing Market Value Share (%), by Type, 2020 and 2031
Figure 33: Rest of Asia Pacific Biologics Contract Manufacturing Market Attractiveness Analysis, by Type, 2021–2031