Analysts’ Viewpoint on Market Scenario
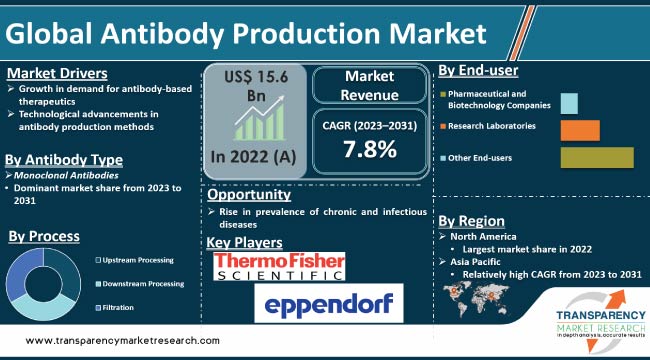
The global antibody production market size is expected to grow at a steady pace during the forecast period due to rise in demand for antibody-based therapeutics. Surge in prevalence of chronic and infectious diseases is also projected to augment market expansion in the next few years.
Growth in investment in research and development activities and technological advancements in antibody production methods are likely to create lucrative opportunities for vendors in the antibody production industry. Vendors are focusing on custom antibody production for biomedical applications. The sector is also benefiting from the surge in demand for personalized medicine and targeted therapies.
Antibody production refers to the process of generating antibodies, which are proteins produced by the immune system to identify and neutralize foreign substances such as bacteria, viruses, and cancer cells. Antibodies have become essential tools in medical research, diagnostics, and therapeutics, with applications in a wide range of fields including cancer treatment, infectious disease diagnosis, and vaccine development.
Antibody production can be achieved through various methods, including hybridoma technology, phage display, and recombinant DNA technology. Increase in demand for personalized medicine, targeted therapies, and biologics is anticipated to spur the antibody production market growth in the near future.
Emergence of the COVID-19 pandemic has led to surge in demand for antibodies for research, diagnosis, and treatment purposes. Numerous biotech and pharmaceutical companies are developing monoclonal antibodies for the treatment of COVID-19.
Increase in prevalence of chronic and infectious diseases and growth in awareness and affordability of personalized medicine and targeted therapies are boosting the demand for antibody-based therapeutics, especially in emerging economies.
Monoclonal antibodies (mAbs) have become the predominant treatment for various diseases in the last 25 years. Technological advances have made the discovery and development of mAb therapies quicker and more efficient. According to the U.S. FDA, as of 2017, there were 61 mAbs in clinical use, globally, with 48 new ones approved since 2008. In 2018 and 2019, 18 new antibodies were approved by the FDA. Thus, rise in adoption of therapeutic mAbs is fueling the antibody production market revenue.
According to an article published in Springer Nature, over 570 monoclonal antibodies (mAbs) are under clinical trials, with most of them in early-stage studies to assess safety and efficacy in cancer treatment. 29 novel mAbs are in late-stage clinical trials for non-cancer indications, with 40% of them intended for immune-mediated disorders. In 2019, five mAbs for cancer treatment were submitted to the FDA for license applications. These developments highlight the continued significance of mAbs as a treatment modality for various diseases, including cancer and immune-mediated disorders.
Technological advancements in antibody production methods have revolutionized the way antibodies are produced, leading to increased efficiency, speed, and precision. These advancements have played a vital role in driving the antibody production market development.
Hybridoma technology has revolutionized various fields, including cell biology, immunology, biotechnology, toxicology, pharmaceutical research, and medical research. It has enabled the production of highly specific and sensitive monoclonal antibodies in large quantities, which have been extensively used in diagnostics and cancer therapy. Prior to hybridoma technology, crude sera from immunized laboratory animals were employed, leading to allergic and hypersensitive reactions in patients.
Moreover, the development of the phage display method and western blotting represents an important milestone in the production of human monoclonal antibodies. This technique enables researchers to identify recombinant monoclonal antibodies against antigens more quickly and without the need for animal immunization. The phage display method allows for the in vitro selection of monoclonal antibodies by genetically engineering bacteriophages and using repeated antigen-guided selection and phage propagation.
Furthermore, the development of high-throughput screening techniques and automation has enabled the rapid screening of several antibodies, allowing researchers to identify potential therapeutic candidates more efficiently.
Monoclonal antibodies was the largest antibody type segment with 40.0% share in 2022. Monoclonal antibodies are being increasingly used in the diagnosis and treatment of various diseases, including cancer, autoimmune disorders, and infectious diseases.
Growth of the monoclonal antibodies segment can be ascribed to the strategic initiatives adopted by pharmaceutical and biotech companies. These include increase in investment in research and development, resulting in product approvals and launches, and growth in collaborations to develop advanced monoclonal antibodies. In May 2021, Cipla launched Roche’s antibody cocktail for the treatment of mild to moderate COVID-19 in India.
Moreover, global pharmaceutical companies are heavily investing in the R&D of cancer biologics drugs, which include monoclonal antibodies, due to their high efficiency and lower toxicity in the diagnosis and treatment of various types of cancer as compared to chemotherapy and other cancer treatment methods. These factors are expected to drive the monoclonal antibodies segment during the forecast period.
According to the latest antibody production market trends, the downstream processing segment is anticipated to dominate the industry during the forecast period. Downstream processing involves the purification and isolation of antibodies from a complex mixture of biological materials, such as fermentation broth or cell culture supernatants. This process is critical to ensure the quality, purity, and potency of the final antibody product. Surge in demand for high-quality and pure antibodies for various research and therapeutic applications is augmenting the downstream processing segment.
Recent advancements in antibody processing have resulted in the development of new technologies that enhance the efficiency, cost-effectiveness, and quality of antibody production. These include single-use sensors, membrane chromatography technology, remote monitoring, and data analytics. Single-use sensors enable real-time monitoring of bioprocess parameters, improving process control and reducing the risk of contamination. Membrane chromatography technology offers a more cost-effective and flexible alternative to traditional resin-based chromatography, thereby reducing the overall manufacturing cost and increasing productivity.
In March 2019, GE Healthcare partnered with Amgen to establish a digital data exchange collaboration program to understand the advances in the bio-manufacturing process on various raw material variability. This collaborative effort aimed to leverage data analytics to improve the efficiency and quality of antibody production, with the ultimate goal of reducing the cost of biologics. Growth in adoption of such advanced processing technologies is estimated to boost the segment in the next few years.
According to the latest antibody production market analysis, the pharmaceutical and biotechnology companies end-user segment is anticipated to dominate the industry during the forecast period. This can be ascribed to increase in demand for biologics and growth in prevalence of chronic diseases such as cancer, autoimmune disorders, and infectious diseases.
Pharmaceutical and biotechnology companies are major consumers of monoclonal antibodies, which are extensively utilized in drug discovery, development, and commercialization.
Moreover, these companies invest heavily in research and development to introduce new and advanced monoclonal antibodies, which can be used for various therapeutic applications. Surge in adoption of personalized and precision medicine approaches is propelling the segment, as monoclonal antibodies are ideal for targeted therapy and precision medicine.
According to the latest antibody production market forecast, North America is expected to hold largest share from 2023 to 2031. Rise in investment in drug discovery and advancements in research and healthcare infrastructure are fueling market dynamics in the region.
North America is home to a robust ecosystem for drug discovery and development, with a large pool of skilled workforce and advanced research facilities. This is prompting the development of several novel and advanced antibody-based products, thereby driving market statistics in the region.
Increase in adoption of biopharmaceuticals is also boosting the production of antibodies in North America. Biopharmaceuticals offer several advantages over traditional small-molecule drugs, including greater specificity, efficacy, and safety, which make them ideal for the treatment of various diseases. Additionally, the adoption of advanced technologies, such as AI technology, is expected to accelerate the drug development process and reduce the cost of discovering new drugs. This is expected to further fuel growth of the antibody production industry in North America in the next few years.
The business in Asia Pacific is projected to grow at a steady pace during the forecast period due to increase in investment in the R&D of new and advanced monoclonal antibodies, polyclonal antibodies, and other antibody-based products. Rise in adoption of antibody-based products for the treatment of various diseases is also fueling market progress in Asia Pacific.
Danaher Corporation, Merck KGaA, Sartorius, Thermo Fisher Scientific Inc., Eppendorf AG, INTEGRA Biosciences AG, Genetix Biotech Asia Pvt Ltd., Solaris Biotech, Grifols, F. Hoffmann-La Roche AG, and FiberCell Systems Inc. are key entities operating in the market.
Vendors are offering high-quality antibody production services for research companies and investing significantly in the R&D of new products to increase their antibody production market share. These vendors have been profiled in the antibody production market report based on parameters such as product portfolio, financial overview, latest developments, business strategies, business segments, and company overview.
| Attribute | Detail |
|---|---|
|
Market Size in 2022 |
US 15.6 Bn |
|
Market Forecast (Value) in 2031 |
US$ 30.7 Bn |
|
Growth Rate (CAGR) |
7.8% |
|
Forecast Period |
2023-2031 |
|
Historical Data Available for |
2017-2022 |
|
Quantitative Units |
US$ Bn for Value |
|
Market Analysis |
It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, Porter’s Five Forces analysis, value chain analysis, and key trend analysis. |
|
Competition Landscape |
|
|
Format |
Electronic (PDF) + Excel |
|
Market Segmentation |
|
|
Regions Covered |
|
|
Countries Covered |
|
|
Companies Profiled |
|
|
Customization Scope |
Available upon request |
|
Pricing |
Available upon request |
The global market was valued at US$ 15.6 Bn in 2022
It is projected to reach US$ 30.7 Bn by the end of 2031
It is projected to be 7.8% from 2023 to 2031
Growth in demand for antibody-based therapeutics and technological advancements in antibody production methods
Monoclonal antibodies was the largest antibody type segment with 40.0% share in 2022
North America is anticipated to record the highest demand from 2023 to 2031
Danaher Corporation, Merck KGaA, Sartorius, Thermo Fisher Scientific Inc., Eppendorf AG, INTEGRA Biosciences AG, Genetix Biotech Asia Pvt Ltd., Solaris Biotech, Grifols, F. Hoffmann-La Roche AG, and FiberCell Systems Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Antibody Production Market
4. Market Overview
4.1. Introduction
4.1.1. Product Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Antibody Production Market Analysis and Forecast, 2017-2031
5. Key Insights
5.1. Technological Advancements
5.2. Key Mergers & Acquisitions
5.3. Key Product/Brand Analysis
5.4. Top 3 Players Operating in Market Space
5.5. COVID-19 Pandemic Impact on Industry (Value Chain and Short/Mid/Long-term Impact)
6. Global Antibody Production Market Analysis and Forecast, by Antibody Type
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Antibody Type, 2017-2031
6.3.1. Monoclonal Antibodies
6.3.2. Polyclonal Antibodies
6.3.3. Other Antibody Types
6.4. Market Attractiveness Analysis, by Antibody Type
7. Global Antibody Production Market Analysis and Forecast, by Process
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Process, 2017-2031
7.3.1. Upstream Processing
7.3.1.1. Bioreactors
7.3.1.1.1. Large-scale Bioreactors
7.3.1.1.2. Single-use Bioreactors
7.3.1.2. Consumables
7.3.2. Downstream Processing
7.3.2.1. Chromatography Systems
7.3.2.2. Chromatography Resins
7.3.3. Filtration
7.3.3.1. Filtration Systems
7.3.3.2. Filtration Consumables & Accessories
7.4. Market Attractiveness Analysis, by Process
8. Global Antibody Production Market Analysis and Forecast, by End-user
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by End-user, 2017-2031
8.3.1. Pharmaceutical and Biotechnology Companies
8.3.2. Research Laboratories
8.3.3. Other End-users
8.4. Market Attractiveness Analysis, by End-user
9. Global Antibody Production Market Analysis and Forecast, by Region
9.1. Key Findings
9.2. Market Value Forecast, by Region, 2017-2031
9.2.1. North America
9.2.2. Europe
9.2.3. Asia Pacific
9.2.4. Latin America
9.2.5. Middle East & Africa
9.3. Market Attractiveness Analysis, by Region
10. North America Antibody Production Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.2. Market Value Forecast, by Antibody Type, 2017-2031
10.2.1. Monoclonal Antibodies
10.2.2. Polyclonal Antibodies
10.2.3. Other Antibody Types
10.3. Market Value Forecast, by Process, 2017-2031
10.3.1. Upstream Processing
10.3.1.1. Bioreactors
10.3.1.1.1. Large-scale Bioreactors
10.3.1.1.2. Single-use Bioreactors
10.3.1.2. Consumables
10.3.2. Downstream Processing
10.3.2.1. Chromatography Systems
10.3.2.2. Chromatography Resins
10.3.3. Filtration
10.3.3.1. Filtration Systems
10.3.3.2. Filtration Consumables & Accessories
10.4. Market Value Forecast, by End-user, 2017-2031
10.4.1. Pharmaceutical and Biotechnology Companies
10.4.2. Research Laboratories
10.4.3. Other End-users
10.5. Market Value Forecast, by Country, 2017-2031
10.5.1. U.S.
10.5.2. Canada
10.6. Market Attractiveness Analysis
10.6.1. By Antibody Type
10.6.2. By Process
10.6.3. By End-user
10.6.4. By Country
11. Europe Antibody Production Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.2. Market Value Forecast, by Antibody Type, 2017-2031
11.2.1. Monoclonal Antibodies
11.2.2. Polyclonal Antibodies
11.2.3. Other Antibody Types
11.3. Market Value Forecast, by Process, 2017-2031
11.3.1. Upstream Processing
11.3.1.1. Bioreactors
11.3.1.1.1. Large-scale Bioreactors
11.3.1.1.2. Single-use Bioreactors
11.3.1.2. Consumables
11.3.2. Downstream Processing
11.3.2.1. Chromatography Systems
11.3.2.2. Chromatography Resins
11.3.3. Filtration
11.3.3.1. Filtration Systems
11.3.3.2. Filtration Consumables & Accessories
11.4. Market Value Forecast, by End-user, 2017-2031
11.4.1. Pharmaceutical and Biotechnology Companies
11.4.2. Research Laboratories
11.4.3. Other End-users
11.5. Market Value Forecast, by Country/Sub-region, 2017-2031
11.5.1. Germany
11.5.2. U.K.
11.5.3. France
11.5.4. Spain
11.5.5. Italy
11.5.6. Rest of Europe
11.6. Market Attractiveness Analysis
11.6.1. By Antibody Type
11.6.2. By Process
11.6.3. By End-user
11.6.4. By Country/Sub-region
12. Asia Pacific Antibody Production Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Antibody Type, 2017-2031
12.2.1. Monoclonal Antibodies
12.2.2. Polyclonal Antibodies
12.2.3. Other Antibody Types
12.3. Market Value Forecast, by Process, 2017-2031
12.3.1. Upstream Processing
12.3.1.1. Bioreactors
12.3.1.1.1. Large-scale Bioreactors
12.3.1.1.2. Single-use Bioreactors
12.3.1.2. Consumables
12.3.2. Downstream Processing
12.3.2.1. Chromatography Systems
12.3.2.2. Chromatography Resins
12.3.3. Filtration
12.3.3.1. Filtration Systems
12.3.3.2. Filtration Consumables & Accessories
12.4. Market Value Forecast, by End-user, 2017-2031
12.4.1. Pharmaceutical and Biotechnology Companies
12.4.2. Research Laboratories
12.4.3. Other End-users
12.5. Market Value Forecast, by Country/Sub-region, 2017-2031
12.5.1. China
12.5.2. Japan
12.5.3. India
12.5.4. Australia & New Zealand
12.5.5. Rest of Asia Pacific
12.6. Market Attractiveness Analysis
12.6.1. By Antibody Type
12.6.2. By Process
12.6.3. By End-user
12.6.4. By Country/Sub-region
13. Latin America Antibody Production Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Antibody Type, 2017-2031
13.2.1. Monoclonal Antibodies
13.2.2. Polyclonal Antibodies
13.2.3. Other Antibody Types
13.3. Market Value Forecast, by Process, 2017-2031
13.3.1. Upstream Processing
13.3.1.1. Bioreactors
13.3.1.1.1. Large-scale Bioreactors
13.3.1.1.2. Single-use Bioreactors
13.3.1.2. Consumables
13.3.2. Downstream Processing
13.3.2.1. Chromatography Systems
13.3.2.2. Chromatography Resins
13.3.3. Filtration
13.3.3.1. Filtration Systems
13.3.3.2. Filtration Consumables & Accessories
13.4. Market Value Forecast, by End-user, 2017-2031
13.4.1. Pharmaceutical and Biotechnology Companies
13.4.2. Research Laboratories
13.4.3. Other End-users
13.5. Market Value Forecast, by Country/Sub-region, 2017-2031
13.5.1. Brazil
13.5.2. Mexico
13.5.3. Rest of Latin America
13.6. Market Attractiveness Analysis
13.6.1. By Antibody Type
13.6.2. By Process
13.6.3. By End-user
13.6.4. By Country/Sub-region
14. Middle East & Africa Antibody Production Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Antibody Type, 2017-2031
14.2.1. Monoclonal Antibodies
14.2.2. Polyclonal Antibodies
14.2.3. Other Antibody Types
14.3. Market Value Forecast, by Process, 2017-2031
14.3.1. Upstream Processing
14.3.1.1. Bioreactors
14.3.1.1.1. Large-scale Bioreactors
14.3.1.1.2. Single-use Bioreactors
14.3.1.2. Consumables
14.3.2. Downstream Processing
14.3.2.1. Chromatography Systems
14.3.2.2. Chromatography Resins
14.3.3. Filtration
14.3.3.1. Filtration Systems
14.3.3.2. Filtration Consumables & Accessories
14.4. Market Value Forecast, by End-user, 2017-2031
14.4.1. Pharmaceutical and Biotechnology Companies
14.4.2. Research Laboratories
14.4.3. Other End-users
14.5. Market Value Forecast, by Country/Sub-region, 2017-2031
14.5.1. GCC Countries
14.5.2. South Africa
14.5.3. Rest of Middle East & Africa
14.6. Market Attractiveness Analysis
14.6.1. By Antibody Type
14.6.2. By Process
14.6.3. By End-user
14.6.4. By Country/Sub-region
15. Competition Landscape
15.1. Market Player - Competition Matrix (By Tier and Size of Companies)
15.2. Market Share Analysis, by Company (2022)
15.3. Company Profiles
15.3.1. Danaher Corporation
15.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.1.2. Product Portfolio
15.3.1.3. Financial Overview
15.3.1.4. SWOT Analysis
15.3.1.5. Strategic Overview
15.3.2. Merck KGaA
15.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.2.2. Product Portfolio
15.3.2.3. Financial Overview
15.3.2.4. SWOT Analysis
15.3.2.5. Strategic Overview
15.3.3. Sartorius
15.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.3.2. Product Portfolio
15.3.3.3. Financial Overview
15.3.3.4. SWOT Analysis
15.3.3.5. Strategic Overview
15.3.4. Thermo Fisher Scientific Inc.
15.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.4.2. Product Portfolio
15.3.4.3. Financial Overview
15.3.4.4. SWOT Analysis
15.3.4.5. Strategic Overview
15.3.5. Eppendorf AG
15.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.5.2. Product Portfolio
15.3.5.3. Financial Overview
15.3.5.4. SWOT Analysis
15.3.5.5. Strategic Overview
15.3.6. INTEGRA Biosciences AG
15.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.6.2. Product Portfolio
15.3.6.3. Financial Overview
15.3.6.4. SWOT Analysis
15.3.6.5. Strategic Overview
15.3.7. Genetix Biotech Asia Pvt Ltd
15.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.7.2. Product Portfolio
15.3.7.3. Financial Overview
15.3.7.4. SWOT Analysis
15.3.7.5. Strategic Overview
15.3.8. Solaris Biotech
15.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.8.2. Product Portfolio
15.3.8.3. Financial Overview
15.3.8.4. SWOT Analysis
15.3.8.5. Strategic Overview
15.3.9. Grifols
15.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.9.2. Product Portfolio
15.3.9.3. Financial Overview
15.3.9.4. SWOT Analysis
15.3.9.5. Strategic Overview
15.3.10. F. Hoffmann-La Roche AG
15.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.10.2. Product Portfolio
15.3.10.3. Financial Overview
15.3.10.4. SWOT Analysis
15.3.10.5. Strategic Overview
15.3.11. FiberCell Systems Inc.
15.3.11.1. Company Overview (HQ, Business Segments, Employee Strength)
15.3.11.2. Product Portfolio
15.3.11.3. Financial Overview
15.3.11.4. SWOT Analysis
15.3.11.5. Strategic Overview
List of Tables
Table 01: Global Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 02: Global Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 03: Global Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
Table 04: Global Antibody Production Market Value (US$ Bn) Forecast, by Region, 2017-2031
Table 05: North America Antibody Production Market Value (US$ Bn) Forecast, by Country, 2017-2031
Table 06: North America Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 07: North America Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 08: North America Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
Table 09: Europe Antibody Production Market Value (US$ Bn) Forecast, by Country/Sub-region, 2017-2031
Table 10: Europe Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 11: Europe Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
Table 12: Europe Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 13: Asia Pacific Antibody Production Market Value (US$ Bn) Forecast, by Country/Sub-region, 2017-2031
Table 14: Asia Pacific Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 15: Asia Pacific Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 16: Asia Pacific Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
Table 17: Latin America Antibody Production Market Value (US$ Bn) Forecast, by Country/Sub-region, 2017-2031
Table 18: Latin America Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 19: Latin America Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 20: Latin America Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
Table 21: Middle East & Africa Antibody Production Market Value (US$ Bn) Forecast, by Country/Sub-region, 2017-2031
Table 22: Middle East & Africa Antibody Production Market Value (US$ Bn) Forecast, by Antibody Type, 2017-2031
Table 23: Middle East & Africa Antibody Production Market Value (US$ Bn) Forecast, by Process, 2017-2031
Table 24: Middle East & Africa Antibody Production Market Value (US$ Bn) Forecast, by End-user, 2017-2031
List of Figures
Figure 01: Global Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 02: Global Antibody Production Market Value Share, by Antibody Type, 2022
Figure 03: Global Antibody Production Market Value Share, by End-user, 2022
Figure 04: Global Antibody Production Market Value Share, by Region, 2022
Figure 05: Global Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 06: Global Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 07: Global Antibody Production Market Revenue (US$ Bn), by Monoclonal Antibodies, 2017-2031
Figure 08: Global Antibody Production Market Revenue (US$ Bn), by Polyclonal Antibodies 2017-2031
Figure 09: Global Antibody Production Market Revenue (US$ Bn), by Other Antibody Types, 2017-2031
Figure 10: Global Antibody Production Market Revenue (US$ Bn), by Process, 2017-2031
Figure 11: Global Antibody Production Market Revenue (US$ Bn), by Upstream Processing 2017-2031
Figure 12: Global Antibody Production Market Revenue (US$ Bn), by Downstream Processing, 2017-2031
Figure 13: Global Antibody Production Market Revenue (US$ Bn), by Filtration, 2017-2031
Figure 14: Global Antibody Production Market Value Share Analysis, by Filtration Systems, 2022 and 2031
Figure 15: Global Antibody Production Market Attractiveness Analysis, by Filtration Consumables & Accessories, 2023-2031
Figure 16: Global Antibody Production Market Revenue (US$ Bn), by End-user, 2017-2031
Figure 17: Global Antibody Production Market Revenue (US$ Bn), Pharmaceutical and Biotechnology Companies, 2017-2031
Figure 18: Global Antibody Production Market Revenue (US$ Bn), by Research Laboratories, 2017-2031
Figure 19: Global Antibody Production Market Revenue (US$ Bn), by Other End-users, 2017-2031
Figure 20: Global Antibody Production Market Value Share Analysis, by Region, 2022 and 2031
Figure 21: Global Antibody Production Market Attractiveness Analysis, by Region, 2023-2031
Figure 22: North America Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 23: North America Antibody Production Market Value Share Analysis, by Country, 2022 and 2031
Figure 24: North America Antibody Production Market Attractiveness Analysis, by Country, 2023-2031
Figure 25: North America Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 26: North America Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 27: North America Antibody Production Market Value Share Analysis (US$ Bn), by End-user, 2017-2031
Figure 28: North America Antibody Production Market Attractiveness Analysis, by End-user, 2023-2031
Figure 31: Europe Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 32: Europe Antibody Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 33: Europe Antibody Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 34: Europe Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 35: Europe Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 36: Europe Antibody Production Market Value Share Analysis, by End-user, 2017-2031
Figure 37: Europe Antibody Production Market Attractiveness Analysis, by End-user, 2023-2031
Figure 40: Asia Pacific Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 41: Asia Pacific Antibody Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 42: Asia Pacific Antibody Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 43: Asia Pacific Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 44: Asia Pacific Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 45: Asia Pacific Antibody Production Market Value Share Analysis, by End-user, 2017-2031
Figure 46: Asia Pacific Antibody Production Market Attractiveness Analysis, by End-user, 2023-2031
Figure 49: Latin America Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 50: Latin America Antibody Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 51: Latin America Antibody Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 52: Latin America Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 53: Latin America Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 54: Latin America Antibody Production Market Value Share Analysis, by End-user, 2017-2031
Figure 55: Latin America Antibody Production Market Attractiveness Analysis, by End-user, 2023-2031
Figure 58: Middle East & Africa Antibody Production Market Value (US$ Bn) Forecast, 2017-2031
Figure 59: Middle East & Africa Antibody Production Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 60: Middle East & Africa Antibody Production Market Attractiveness Analysis, by Country/Sub-region, 2023-2031
Figure 61: Middle East & Africa Antibody Production Market Value Share Analysis, by Antibody Type, 2022 and 2031
Figure 62: Middle East & Africa Antibody Production Market Attractiveness Analysis, by Antibody Type, 2023-2031
Figure 63: Middle East & Africa Antibody Production Market Value Share Analysis, by End-user, 2017-2031
Figure 64: Middle East & Africa Antibody Production Market Attractiveness Analysis, by End-user, 2023-2031