The Global Active Implantable Medical Devices Market: Overview
Few of the most notable and prominent driving factors positively influencing the demand dynamics within the global active implantable medical devices market include rise in the elderly and aging global population, rising funds and investments intended for development of the technically advanced solutions, favorable reimbursement scenario for ear, nose, and throat or ENT procedures in developed economies, rising prevalence of neurological as well as cardiovascular disorders, and rising applications of neurostimulators. On the other hand, problems related to usage of implantable medical devices, high cost of these implants, and unfavorable healthcare policies in developed countries such as the United States may restrict the growth within the global active implantable medical devices market in coming years.
Key types of products offered by the players in the global active implantable medical devices market include implantable cardioverter defibrillators, ventricular assist devices, implantable heart monitors, neurostimulators, insert able loop recorders, and implantable hearing devices, including passive or non-active hearing implants and active hearing implants. Implantable cardioverter defibrillators are further classified into subcutaneous implantable cardioverter defibrillators and trans venous implantable cardioverter defibrillators including biventricular implantable cardioverter defibrillators, dual chamber implantable cardioverter defibrillators, cardiac resynchronization therapy defibrillators, and single chamber implantable cardioverter defibrillators. Types of neurostimulators products offered by the players in global active implantable medical devices market include deep brain stimulators, vagus nerve stimulators, spinal cord stimulators, sacral nerve stimulators, and gastric electrical stimulators.
Major and leading players and manufacturers operational within the global active implantable medical devices market are adopting various corporate growth strategies in order to amass a major share in the global active implantable medical devices market in coming years. Some of the key strategies employed by these vendors in the global active implantable medical devices market include new product launches, agreements and collaborations, strategic partnerships, mergers and acquisitions, and geographical expansion. The global active implantable medical devices market was led by three major players in the last few years, namely, Medtronic PLC, Boston Scientific Corporation, and Abbott Laboratories. Together, these three players are thought to account for 95 % of the industry share.
Active Implantable Medical Devices Market: Snapshot
The global market for active implantable medical devices is gaining remarkably from the increasing prevalence of several chronic diseases, such as cardiovascular and neurological disorders. The constant product launches, and simplified approval process is also supporting the growth of this market substantially.
Going forward, the rising expenditure on healthcare, owing to the increasing number of patients with cardiovascular diseases and neurological disorders, is anticipated to fuel the demand for active implantable medical devices across the world over the next few years. However, the issues regarding the usage of active implantable medical devices, such as device failure and concerns related to cybersecurity may hinder the growth of the market in the near future. The global market for active implantable medical devices is likely to present an opportunity worth US$20.73 bn by 2025, expanding at a CAGR of 4.40% between 2017 and 2025.
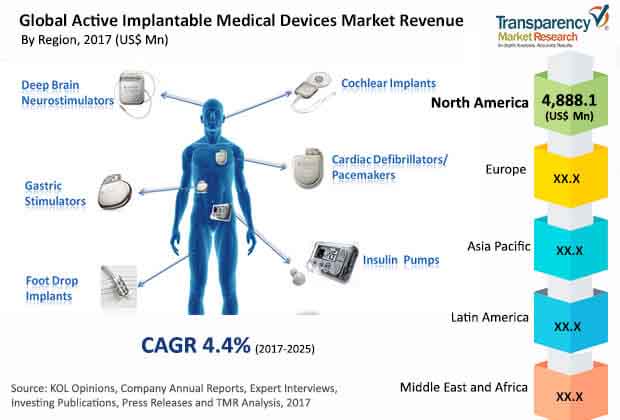
Demand for Implantable Cardioverter Defibrillators to Remain Strong
The global active implantable medical devices market is assessed on the basis of the product type, procedure, and the end user. Based on the product type, the market is classified into implantable cardioverter defibrillators (ICD), cardiac pacemaker, nerve stimulators, cochlear implants, ventricular assist devices (VAD), and insertable cardiac monitor (implantable monitoring devices). Among these, the demand for implantable cardioverter defibrillators (ICD) is greater at present and is expected to remain so over the next few years. However, insertable cardiac monitors are expected to register a robust rise in their demand in the near future, thanks to the increasing prevalence of cardiovascular diseases across the world.
Based on the procedure, the market is categorized into cardiovascular implants, neurological implants, and hearing implants. By the end user, the market is divided into hospitals, ambulatory surgery centers, and specialty clinics. With significantly rising investments, the hospital sector is likely to emerge as the most prominent end user of active implantable medical devices in the years to come.
Presence of Leading Players to Ensure North America’s Lead
Asia Pacific, Latin America, North America, Europe, and the Middle East Africa are the main geographical segments of the worldwide market for active implantable medical devices. With a share of more than 33%, North America dominated the global market in 2016, thanks to the technological advancements in developing novel active implantable devices. On account of the considerable presence of a number of implantable medical device manufacturers, such as Medtronic Plc, Boston Scientific Corp., and Abbott Laboratories, this regional market is anticipated to remain on the top over the forthcoming years.
Europe, with a share of 28.4%, stood at the second position in 2016. It is expected to retain its position over the next few years, thanks to the frequent product launches. Asia Pacific, however, is projected to present the most promising growth opportunities to market players in the near future, owing to the remarkable rise in Asian economies, such as China, India, and South Korea, leading to increase in investments by implantable medical device manufacturers.
Boston Scientific Corp., Abbott Laboratories, Medtronic Plc, Sonova Holding AG, LivaNova Plc, BIOTRONIK SE & Co. KG, Cochlear Ltd., William Demant Holding A/S, Nurotron Biotechnology Co. Ltd., and MED-EL Medical Electronics are some of the leading vendors of active implantable medical devices across the world. The market is demonstrating a highly competitive landscape and is expected remain intensely competitive over the forthcoming years.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary : Global Active Implantable Medical Devices Market
4. Market Overview
4.1. Introduction
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Active Implantable Medical Devices Market Analysis and Forecasts, 2017–2025
4.5. Porter’s Five Force Analysis
4.6. Market Outlook
5. Global Active Implantable Medical Devices Market Analysis and Forecasts, By Product
5.1. Introduction & Definition
5.2. Key Findings / Developments
5.3. Key Trends
5.4. Market Value Forecast By Product
5.4.1. Cardiac Pacemaker
5.4.1.1. Single Chamber Pacemaker
5.4.1.2. Dual Chamber Pacemaker
5.4.1.3. Others
5.4.2. Implantable Cardioverter Defibrillators (ICD)
5.4.2.1. Transvenous Implantable Cardioverter Defibrillators
5.4.2.2. Subcutaneous Implantable Cardioverter Defibrillators
5.4.3. Nerve Stimulators
5.4.4. Cochlear Implants
5.4.5. Ventricular Assist Devices (VAD)
5.4.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
5.5. Market Attractiveness By Product
6. Global Active Implantable Medical Devices Market Analysis and Forecasts, By Procedure
6.1. Introduction & Definition
6.2. Key Findings / Developments
6.3. Key Trends
6.4. Market Value Forecast By Procedure
6.4.1. Cardiovascular Implants
6.4.2. Neurological Implants
6.4.3. Hearing Implants
6.4.4. Others
6.5. Market Attractiveness By Procedure
7. Global Active Implantable Medical Devices Market Analysis and Forecasts, By End-User
7.1. Introduction & Definition
7.2. Key Findings / Developments
7.3. Key Trends
7.4. Market Value Forecast By End-User
7.4.1. Hospitals
7.4.2. Ambulatory Surgery Centers
7.4.3. Specialty Clinics
7.5. Market Attractiveness By End-User
8. Global Active Implantable Medical Devices Market Analysis and Forecasts, By Region
8.1. Key Findings
8.2. Market Value Forecast By Region
8.2.1. North America
8.2.2. Europe
8.2.3. Asia Pacific
8.2.4. Latin America
8.2.5. Middle East and Africa
8.3. Market Attractiveness By Country/Region
9.North America Active Implantable Medical Devices Market Analysis and Forecast
9.1. Introduction
9.1.1. Key Findings
9.1.2. Key Trends
9.2. Market Value Forecast By Product
9.2.1. Cardiac Pacemaker
9.2.1.1. Single Chamber Pacemaker
9.2.1.2. Dual Chamber Pacemaker
9.2.1.3. Others
9.2.2. Implantable Cardioverter Defibrillators (ICD)
9.2.2.1. Transvenous Implantable Cardioverter Defibrillators
9.2.2.2. Subcutaneous Implantable Cardioverter Defibrillators
9.2.3. Nerve Stimulators
9.2.4. Cochlear Implants
9.2.5. Ventricular Assist Devices (VAD)
9.2.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
9.3. Market Value Forecast By Procedure
9.3.1. Cardiovascular Implants
9.3.2. Neurological Implants
9.3.3. Hearing Implants
9.3.3. Others
9.4. Market Value Forecast By End-User
9.4.1. Hospitals
9.4.2. Ambulatory Surgery Centers
9.4.3. Specialty Clinics
9.5. Market Value Forecast By Country
9.5.1. U.S.
9.5.2. Canada
9.6. Market Attractiveness Analysis
10. Europe Active Implantable Medical Devices Market Analysis and Forecast
10.1. Introduction
10.1.1. Key Findings
10.1.2. Key Trends
10.2. Market Value Forecast By Product
10.2.1. Cardiac Pacemaker
10.2.1.1. Single Chamber Pacemaker
10.2.1.2. Dual Chamber Pacemaker
10.2.1.3. Others
10.2.2. Implantable Cardioverter Defibrillators (ICD)
10.2.2.1. Transvenous Implantable Cardioverter Defibrillators
10.2.2.2. Subcutaneous Implantable Cardioverter Defibrillators
10.2.3. Nerve Stimulators
10.2.4. Cochlear Implants
10.2.5. Ventricular Assist Devices (VAD)
10.2.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
10.3. Market Value Forecast By Procedure
10.3.1 Cardiovascular Implants
10.3.2.Neurological Implants
10.3.3.Hearing Implants
10.3.4.Others
10.4. Market Value Forecast By End-User , 2015–2025
10.4.1. Hospitals
10.4.2. Ambulatory Surgery Centers
10.4.3. Specialty Clinics
10.5. Market Value Forecast By Country , 2015–2025
10.5.1. Germany
10.5.2. France
10.5.3. U.K.
10.5.4. Italy
10.5.4. Spain
10.5.4. Rest of Europe
10.6. Market Attractiveness Analysis
11. Asia Pacific Active Implantable Medical Devices Market Analysis and Forecast
11.1. Introduction
11.1.1. Key Findings
11.1.2. Key Trends
11.2. Market Value Forecast By Product
11.2.1. Cardiac Pacemaker
11.2.1.1. Single Chamber Pacemaker
11.2.1.2. Dual Chamber Pacemaker
11.2.1.3. Others
11.2.2. Implantable Cardioverter Defibrillators (ICD)
11.2.2.1. Transvenous Implantable Cardioverter Defibrillators
11.2.2.2. Subcutaneous Implantable Cardioverter Defibrillators
11.2.3. Nerve Stimulators
11.2.4. Cochlear Implants
11.2.5. Ventricular Assist Devices (VAD)
11.2.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
11.3. Market Value Forecast By Procedure
11.3.1 Cardiovascular Implants
11.3.2.Neurological Implants
11.3.3.Hearing Implants
11.3.4.Others
11.4. Market Value Forecast By End-User
11.4.1. Hospitals
11.4.2. Ambulatory Surgery Centers
11.4.3. Specialty Clinics
11.5. Market Value Forecast By Country
11.5.1. Japan
11.5.2. China
11.5.3. India
11.5.4. Australia
11.5.5. Rest of Asia Pacific
11.6. Market Attractiveness Analysis
12. Latin America Active Implantable Medical Devices Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.1.2. Key Trends
12.2. Market Value Forecast By Product
12.2.1. Cardiac Pacemaker
12.2.1.1. Single Chamber Pacemaker
12.2.1.2. Dual Chamber Pacemaker
12.2.1.3. Others
12.2.2. Implantable Cardioverter Defibrillators (ICD)
12.2.2.1. Transvenous Implantable Cardioverter Defibrillators
12.2.2.2. Subcutaneous Implantable Cardioverter Defibrillators
12.2.3. Nerve Stimulators
12.2.4. Cochlear Implants
12.2.5. Ventricular Assist Devices (VAD)
12.2.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
12.3. Market Value Forecast By Procedure
12.3.1 Cardiovascular Implants
12.3.2.Neurological Implants
12.3.3.Hearing Implants
12.3.4.Others
12.4. Market Value Forecast By End-User
12.4.1. Hospitals
12.4.2. Ambulatory Surgery Centers
12.4.3. Specialty Clinics
12.5. Market Value Forecast By Country
12.5.1. Brazil
12.5.2. Mexico
12.5.3. Rest of Latin America
12.6. Market Attractiveness Analysis
13. Middle East and Africa Active Implantable Medical Devices Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.1.2. Key Trends
13.2. Market Value Forecast By Product
13.2.1. Cardiac Pacemaker
13.2.1.1. Single Chamber Pacemaker
13.2.1.2. Dual Chamber Pacemaker
13.2.1.3. Others
13.2.2. Implantable Cardioverter Defibrillators (ICD)
13.2.2.1. Transvenous Implantable Cardioverter Defibrillators
13.2.2.2. Subcutaneous Implantable Cardioverter Defibrillators
13.2.3. Nerve Stimulators
13.2.4. Cochlear Implants
13.2.5. Ventricular Assist Devices (VAD)
13.2.6. Insertable Cardiac Monitor (Implantable Monitoring Devices)
13.3. Market Value Forecast By Procedure
13.3.1 Cardiovascular Implants
13.3.2.Neurological Implants
13.3.3.Hearing Implants
13.3.4.Others
13.4. Market Value Forecast By End-User
13.4.1. Hospitals
13.4.2. Ambulatory Surgery Centers
13.4.3. Specialty Clinics
13.5. Market Value Forecast By Country
13.5.1. South Africa
13.5.2. Israel
13.5.3. GCC countries
13.5.4. Rest of MEA
13.6. Market Attractiveness Analysis
14. Competition Landscape
14.1. Market Player – Competition Matrix (By Tier and Size of companies)
14.2. Market Share Analysis By Company (2016)
14.3. Company Profiles (Details – Overview, Financials, Recent Developments, Strategy)
14.3.1 Abbott Laboratories
14.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.1.2. Business Overview
14.3.1.3. Product Portfolio
14.3.1.4. Financial Overview
14.3.1.5. SWOT Analysis
14.3.1.6. Strategic overview
14.3.2. BIOTRONIK SE & Co. KG
14.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.2.2. Business Overview
14.3.2.3. Product Portfolio
14.3.2.4. Financial Overview
14.3.2.5. SWOT Analysis
14.3.2.6. Strategic overview
14.3.3. Boston Scientific Corporation
14.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.3.2. Business Overview
14.3.3.3. Product Portfolio
14.3.3.4. Financial Overview
14.3.3.5. SWOT Analysis
14.3.3.6. Strategic overview
14.3.4. Cochlear Ltd
14.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.4.2. Business Overview
14.3.4.3. Product Portfolio
14.3.4.4. Financial Overview
14.3.4.5. SWOT Analysis
14.3.4.6. Strategic overview
14.3.5. Sonova Holding AG
14.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.5.2. Business Overview
14.3.5.3. Product Portfolio
14.3.5.4. Financial Overview
14.3.5.5. SWOT Analysis
14.3.5.6. Strategic overview
14.3.6. LivaNova PLC
14.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.6.2. Business Overview
14.3.6.3. Product Portfolio
14.3.6.4. Financial Overview
14.3.6.5. SWOT Analysis
14.3.6.6. Strategic overview
14.3.7. Medtronic Plc.
14.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.7.2. Business Overview
14.3.7.3. Product Portfolio
14.3.7.4. Financial Overview
14.3.7.5. SWOT Analysis
14.3.7.6. Strategic overview
14.3.8. MED-EL Medical Electronics
14.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.8.2. Business Overview
14.3.8.3. Product Portfolio
14.3.8.4. Financial Overview
14.3.8.5. SWOT Analysis
14.3.8.6. Strategic overview
14.3.9. Nurotron Biotechnology Co. Ltd
14.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.9.2. Business Overview
14.3.9.3. Product Portfolio
14.3.9.4. Financial Overview
14.3.9.5. SWOT Analysis
14.3.9.6. Strategic overview
14.3.10. William Demant Holding A/S
14.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
14.3.10.2. Business Overview
14.3.10.3. Product Portfolio
14.3.10.4. Financial Overview
14.3.10.5. SWOT Analysis
14.3.10.6. Strategic overview
List of Tables
Table 01: Global Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 02: Global Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 03: Global Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 04: Global Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure Type, 2017–2025
Table 05: Global Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 06: Global Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Region, 2017–2025
Table 07: North America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product, 2017–2025
Table 08: North America Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 09: North America Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 10: North America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure, 2017–2025
Table 11: North America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 12: North America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Country, 2017–2025
Table 13: Europe Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product, 2017–2025
Table 14: Europe Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 15: Europe Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 16: Europe Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure, 2017–2025
Table 17: Europe Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 18: Europe Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Country, 2017–2025
Table 19: Asia Pacific Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product, 2017–2025
Table 20: Asia Pacific Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 21: Asia Pacific Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 22: Asia Pacific Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure, 2017–2025
Table 23: Asia Pacific Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 24: Asia Pacific Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Country, 2017–2025
Table 25: Latin America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product, 2017–2025
Table 26: Latin America Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 27: Latin America Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 28: Latin America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure, 2017–2025
Table 29: Latin America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 30: Latin America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Country, 2017–2025
Table 31: Middle East and Africa Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Product, 2017–2025
Table 32: Middle East and Africa Cardiac Pacemaker Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 33: Middle East and Africa Implantable Cardioverter Defibrillators (ICD) Market Size (US$ Mn) Forecast, by Product Type, 2017–2025
Table 34: Middle East and Africa Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Procedure, 2017–2025
Table 35: Middle East and Africa Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by End user, 2017–2025
Table 36: Middle East and Africa Active Implantable Medical Devices Market Size (US$ Mn) Forecast, by Country, 2017–2025
List of Figures
Figure 01: Global Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2017–2025
Figure 02: Global Active Implantable Medical Devices Market Value Share Analysis, by Product Type, 2017 and 2025
Figure 03: Global Cardiac Pacemaker Market Revenue (US$ Mn), 2015-2025
Figure 04: Global Implantable Cardioverter Defibrillators Market Revenue, US$ Mn, 2015–2025
Figure 05: Global Nerve Stimulators Market Revenue (US$ Mn), 2015-2025
Figure 06: Global Insertable Cardiac Monitor Market Revenue, US$ Mn, 2015–2025
Figure 07: Global Cochlear Implants Market Revenue (US$ Mn), 2015-2025
Figure 08: Global Ventricular Assist Devices (VAD) Market Revenue, US$ Mn, 2015–2025
Figure 09: Active Implantable Medical Devices Market Attractiveness Analysis By Product Type
Figure 10: Global Active Implantable Medical Devices Market Value Share Analysis By Procedure Type, 2016 and 2024
Figure 07: Cardiovascular Implants Market Revenue, US$ Mn, 2015–2025
Figure 08: Neurological Implants Market Revenue, US$ Mn, 2015–2025
Figure 07: Hearing implants Market Revenue, US$ Mn, 2015–2025
Figure 08: Other Implants Market Revenue, US$ Mn, 2015–2025
Figure 10: Global Active Implantable Medical Devices Market Attractiveness Analysis, By Procedure Type
Figure 11: Global Active Implantable Medical Devices Market Value Share Analysis By End users, 2016 and 2024
Figure 12: Global Hospitals Market Revenue (US$ Mn), 2015–2025
Figure 13: Global Ambulatory Surgery Centers Market Revenue (US$ Mn), 2015–2025
Figure 14: Global Academic & Research Institutes Market Revenue (US$ Mn), 2015–2025
Figure 15: Active Implantable Medical Devices Market Attractiveness Analysis, by End user
Figure 16: Global Active Implantable Medical Devices Market Analysis By Region, 2017 and 2025
Figure 17: Global Active Implantable Medical Devices Market Attractiveness Analysis, By Region Type
Figure 18: North America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2015–2025
Figure 19: North America Market Attractiveness Analysis By Country
Figure 20: North America Active Implantable Medical Devices Market Value Share Analysis By Product Type, 2017 and 2025
Figure 21: North America Active Implantable Medical Devices Market Value Share Analysis By Procedure Type, 2017 and 2025
Figure 22: North America Active Implantable Medical Devices Market Value Share Analysis By End User Type, 2017 and 2025
Figure 23: North America Market Value Share Analysis By Country, 2017 and 2025
Figure 24: North America Market Attractiveness Analysis, by Product
Figure 25: North America Market Attractiveness Analysis, by Procedure
Figure 26: North America Market Attractiveness Analysis, by End User
Figure 27: Europe Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2015–2025
Figure 28: Europe Market Attractiveness Analysis, by Country
Figure 29: Europe Market Value Share Analysis By Product Type, 2017 and 2025
Figure 30: Europe Market Value Share Analysis By Procedure Type, 2017 and 2025
Figure 31: Europe Active Implantable Medical Devices Market Value Share Analysis By End User Type, 2017 and 2025
Figure 32: Europe Market Value Share Analysis, by Country, 2017 and 2025
Figure 33: Europe Market Attractiveness Analysis, by Product
Figure 34: Europe Market Attractiveness Analysis, by Procedure
Figure 35: Europe Market Attractiveness Analysis, by End User
Figure 36: Asia Pacific Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2015–2025
Figure 37: Asia Pacific Market Attractiveness Analysis, by Country
Figure 38: Asia Pacific Market Value Share Analysis By Product Type, 2017 and 2025
Figure 39: Asia Pacific Market Value Share Analysis By Procedure Type, 2017 and 2025
Figure 40: Asia Pacific Active Implantable Medical Devices Market Value Share Analysis By End User Type, 2017 and 2025
Figure 41: Asia Pacific Market Value Share Analysis, by Country, 2017 and 2025
Figure 42: Asia Pacific Market Attractiveness Analysis, by Product
Figure 43: Asia Pacific Market Attractiveness Analysis, by Procedure
Figure 44: Asia Pacific Market Attractiveness Analysis, by End User
Figure 45: Latin America Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2015–2025
Figure 46: Latin America Market Attractiveness Analysis, by Country
Figure 47: Latin America Market Value Share Analysis By Product Type, 2017 and 2025
Figure 48: Latin America Market Value Share Analysis By Procedure Type, 2017 and 2025
Figure 49: Latin America Active Implantable Medical Devices Market Value Share Analysis By End User Type, 2017 and 2025
Figure 50: Latin America Market Value Share Analysis, by Country, 2017 and 2025
Figure 51: Latin America Market Attractiveness Analysis, by Product
Figure 52: Latin America Market Attractiveness Analysis, by Procedure
Figure 53: Latin America Market Attractiveness Analysis, by End User
Figure 54: Middle East and Africa Active Implantable Medical Devices Market Size (US$ Mn) Forecast, 2015–2025
Figure 55: Middle East and Africa Market Attractiveness Analysis, by Country
Figure 56: Middle East and Africa Market Value Share Analysis By Product Type, 2017 and 2025
Figure 57: Middle East and Africa Market Value Share Analysis By Procedure Type, 2017 and 2025
Figure 58: Middle East and Africa Active Implantable Medical Devices Market Value Share Analysis By End User Type, 2017 and 2025
Figure 59: Middle East and Africa Market Value Share Analysis, by Country, 2017 and 2025
Figure 60: Middle East and Africa Market Attractiveness Analysis, by Product
Figure 61: Middle East and Africa Market Attractiveness Analysis, by Procedure
Figure 62: Middle East and Africa Market Attractiveness Analysis, by End User
Figure 63: Global Active Implantable Medical Devices Market Share Analysis By Cardiac Rhythm Management (2016)
Figure 64: Global Active Implantable Medical Devices Market Share Analysis By Neuromodulation (2016)
Figure 65: Global Active Implantable Medical Devices Market Share Analysis By Cochlear Implants (2016)