Analyst Viewpoint
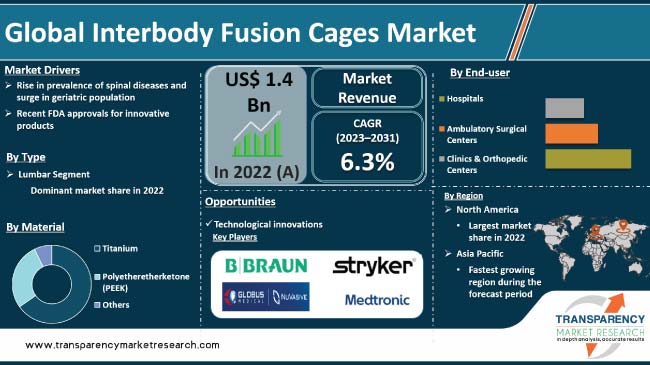
Rise in prevalence of spine-related diseases is driving the global interbody fusion cages market. These devices serve as stabilizers, distributing forces between vertebral bodies and restoring intervertebral and foramina space. Increase in geriatric population and rise in obesity cases across the globe are the other major factors propelling market expansion. Furthermore, recent FDA approvals for innovative products is expected to bolster the global interbody fusion cages industry size during the forecast period.
Introduction of technologically advanced interbody fusion cages offers lucrative opportunities to market players. Companies are investing significantly in 3D printing technology for implant production and development of interbody implants for spinal fusion in order to diversify revenue streams.
Interbody fusion cages are medical devices used in spinal surgery to facilitate the fusion of adjacent vertebrae, providing stability and promoting bone growth between the vertebral bodies. This surgical technique is commonly employed to treat various spinal conditions, including degenerative disc disease, spinal deformities, and instability.
Interbody fusion cages have revolutionized spinal fusion procedures, offering an alternative to traditional bone grafts. These cages are typically inserted into the intervertebral space, replacing the damaged or removed disc. The design of interbody fusion cages allows for proper spinal alignment and provides structural support, addressing issues such as disc height loss and spinal misalignment.
The primary goals of using interbody fusion cages are restoring disc height, decompressing nerve roots, and promoting spinal stability. Surgeons could choose from different types of cages, such as anterior, posterior, or lateral interbody cages, based on the specific requirements of the surgical procedure and the patient's condition. As advancements in spinal surgery continue, interbody fusion cages remain pivotal in enhancing patient outcomes and contributing to the evolution of minimally invasive spinal procedures.
Recent approvals by the U.S. FDA have the potential to significantly drive global interbody fusion cages market demand. The FDA plays a critical role in regulating medical devices, ensuring their safety and efficacy before they can be brought to market.
In May 2023, HAPPE Spine, a medical device firm focusing on developing innovative materials to orthopedic implants, announced that its INTEGRATE-C Interbody Fusion System received FDA 510k clearance.
NTEGRATE-C, powered by the innovative HAPPE platform, stands out as the pioneering interbody fusion cage that offers complete integration of porosity and hydroxyapatite, creating an optimal environment for superior healing.
In May 2023, CTL Amedica Corporation, world's exclusive provider of innovative spine products, received 510k clearance from the FDA) to market the NITRO Interbody Fusion Cage System Family, made entirely of the fusion biomaterial silicon nitride. The products showcase distinct biomaterial properties, incorporating innovative enhancements for the bacteriostatic and osteogenic response.
In May 2022, Accelus, a prominent player in expandable spinal implant technology, received 510(k) clearance from the FDA for its FlareHawk TiHawk 11 Interbody Fusion System. TiHawk11 boasts an insertion profile width of 11mm, expanding to 17mm in width and 14mm in height. This expansion results in a 70% larger footprint compared to a 10mm-wide interbody device of the same length. The enhanced interbody footprint is strategically designed to enhance stability and facilitate post-packing of bone grafts after expansion, thereby increasing graft volume.
Rapid rise in the geriatric population is increasing the incidence of degenerative spine conditions, which, in turn, is fueling demand for advanced spinal solutions.
Spinal diseases, including degenerative disc diseases and spinal deformities, are becoming more prevalent, necessitating effective interventions such as interbody fusion surgeries. The geriatric demographic, in particular, is more susceptible to these conditions due to age-related degeneration, leading to an upsurge in surgical procedures involving interbody fusion cages.
The geriatric population often seeks medical interventions to maintain or improve their quality of life, driving the adoption of advanced technologies such as interbody fusion cages. These devices play a crucial role in enhancing spinal stability, reducing pain, and improving overall patient outcomes.
The interbody fusion cages industry is poised to expand as healthcare providers increasingly incorporate these innovative solutions into their treatment protocols to address the growing challenges posed by spinal diseases in an aging global population. This trend underscores the importance of technological advancements in spinal healthcare to meet the evolving needs of patients and healthcare professionals alike.
In terms of type, the lumbar segment accounted for the largest global interbody fusion cages market share in 2022. Lumbar spine disorders, including, herniated discs, DDD, and spinal stenosis, are frequent conditions affecting large number of individuals globally.
Lumbar interbody fusion cages are specifically designed to address these conditions, making them the preferred choice for surgeons and patients. These disorders cause significant pain, discomfort, and loss of function for individuals, leading to a high demand for effective treatment options.
Interbody fusion cages specifically designed for the lumbar spine are widely used in surgical interventions to address these conditions, contributing to the segment’s dominance.
According to the latest Interbody Fusion Cages market trends, the polyetheretherketone (PEEK) material segment is projected to dominate the industry from 2023 to 2031. PEEK is a biocompatible material widely used in medical implants. It has excellent mechanical properties, including high strength and stiffness, which make it suitable for interbody fusion cages.
PEEK cages provide stability to the spinal column, promoting fusion between vertebral bodies. The material's biocompatibility and stability have led to its increased adoption and dominance in the global interbody fusion cages market.
PEEK is radiolucent, meaning it does not interfere with imaging techniques such as X-rays and magnetic resonance imaging (MRI). This property allows for clear visualization of the fusion site during postoperative monitoring and follow-up evaluations.
Surgeons and healthcare professionals can assess fusion progress and make informed decisions based on accurate imaging, driving the preference for PEEK interbody fusion cages.
PEEK is a versatile material that can be easily machined and customized to match patient anatomy and surgical requirements. Surgeons can select from various sizes and shapes of PEEK interbody fusion cages, allowing for personalized treatment approaches.
The ability to tailor PEEK cages to individual patients' needs enhances surgical outcomes and patient satisfaction, further driving the dominance of the segment. Also, PEEK has a similar modulus of elasticity to bone, which helps reduce stress shielding. Stress shielding occurs when the implant absorbs most of the mechanical stress, leading to bone resorption and decreased bone density.
North America dominated the global interbody fusion cages market in 2023. This can be ascribed to expansion in the microelectronic medical implants market, rise in prevalence of spinal diseases & obesity, and increase in number of spinal implant procedures in the region. For instance, as per information published by American Academy of Orthopaedic Surgeons, 4% to 6% of the U.S. citizens have spondylolisthesis and spondylolysis.
Successful launch of innovative products is also driving the interbody fusion cages market in North America. For instance, in August, 2020, Zavation Medical Products, the U.S based manufacturer of advanced spinal implants announced the launch of the Titanium/PEEK Posterior Lateral Expandable Interbody Fusion (LEIF) cage. The cage is manufactured to offer structural stability with a design suitable for either a posterior or transforaminal approach to the lumbar spine. It comes in diverse heights and geometric configurations, ensuring it aligns with the anatomical requirements of a broad spectrum of patients.
According to interbody fusion cages market forecast, the industry in Asia Pacific is expected to grow at a rapid pace during the forecast period. This is ascribed to large patient population in countries such as India and China, improvement in healthcare facilities, and rise in awareness about spinal diseases and treatments. Additionally, surge in geriatric population is fueling demand for interbody fusion cages in Asia Pacific.
The global interbody fusion cages market is consolidated, with small number of key players accounting for majority share. Companies are investing significantly in R&D to expand product portfolio. Collaborations, partnerships, and mergers & acquisitions are key strategies adopted by interbody fusion cages manufacturers.
Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, B. Braun Melsungen AG, NuVasive, Inc., Alphatec Holdings, Inc., LASAK s.r.o., Xtant Medical Holdings, Inc., and Spineology, Inc. are the prominent players in the market.
Key companies have been profiled in the interbody fusion cages market report based on parameters such as company overview, financial overview, business strategies, product portfolio, business segments, and recent developments.
| Attribute | Detail |
|---|---|
| Size in 2022 | US$ 1.4 Bn |
| Forecast (Value) in 2031 | US$ 2.5 Bn |
| Growth Rate (CAGR) | 6.3% |
| Forecast Period | 2023-2031 |
| Historical Data Available for | 2017-2021 |
| Quantitative Units | US$ Bn for Value |
| Market Analysis | It includes segment analysis as well as regional level analysis. Moreover, qualitative analysis includes drivers, restraints, opportunities, key trends, and parent industry overview. |
| Competition Landscape |
|
| Format | Electronic (PDF) + Excel |
| Market Segmentation |
|
| Regions Covered |
|
| Companies Profiled |
|
| Customization Scope | Available upon request |
| Pricing | Available upon request |
It was valued at US$ 1.4 Bn in 2022
It is projected to reach US$ 2.5 Bn by 2031
It is anticipated to grow at a CAGR of 6.3% from 2023 to 2031
Rise in prevalence of spinal diseases & surge in geriatric population and recent FDA approvals for innovative products
The lumbar type segment accounted for largest share in 2022
North America accounted for major share during the forecast period
Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet, B. Braun Melsungen AG, NuVasive, Inc., Alphatec Holdings, Inc., LASAK s.r.o., Xtant Medical Holdings, Inc., and Spineology, Inc.
1. Preface
1.1. Market Definition and Scope
1.2. Market Segmentation
1.3. Key Research Objectives
1.4. Research Highlights
2. Assumptions and Research Methodology
3. Executive Summary: Global Interbody Fusion Cages Market
4. Market Overview
4.1. Introduction
4.1.1. Type Definition
4.1.2. Industry Evolution / Developments
4.2. Overview
4.3. Market Dynamics
4.3.1. Drivers
4.3.2. Restraints
4.3.3. Opportunities
4.4. Global Interbody Fusion Cages Market Analysis and Forecast, 2017–2031
5. Key Insights
5.1. Regulatory Scenario by Region/Globally
5.2. Key Industry Events (Key Mergers & Acquisitions )
5.3. Disease Epidemiology
5.4. COVID-19 Impact Analysis
6. Global Interbody Fusion Cages Market Analysis and Forecast, by Type
6.1. Introduction & Definition
6.2. Key Findings/Developments
6.3. Market Value Forecast, by Type, 2017–2031
6.3.1. Cervical
6.3.2. Lumbar
6.3.3. Others
6.4. Market Attractiveness Analysis, by Type
7. Global Interbody Fusion Cages Market Analysis and Forecast, by Material
7.1. Introduction & Definition
7.2. Key Findings/Developments
7.3. Market Value Forecast, by Material, 2017–2031
7.3.1. Titanium
7.3.2. Polyetheretherketone (PEEK)
7.3.3. Others
7.4. Market Attractiveness Analysis, by Material
8. Global Interbody Fusion Cages Market Analysis and Forecast, by Product
8.1. Introduction & Definition
8.2. Key Findings/Developments
8.3. Market Value Forecast, by Product, 2017–2031
8.3.1. Expandable Cages
8.3.2. Static Cages
8.4. Market Attractiveness Analysis, by Product
9. Global Interbody Fusion Cages Market Analysis and Forecast, by Indication
9.1. Introduction & Definition
9.2. Key Findings/Developments
9.3. Market Value Forecast, by Indication, 2017–2031
9.3.1. Degenerative Disc Disease
9.3.2. Spondylolisthesis
9.3.3. Spinal Tumors or Masse
9.3.4. Spinal Stenosis
9.3.5. Others
9.4. Market Attractiveness Analysis, by Indication
10. Global Interbody Fusion Cages Market Analysis and Forecast, by End-user
10.1. Introduction & Definition
10.2. Key Findings/Developments
10.3. Market Value Forecast, by End-user, 2017–2031
10.3.1. Hospitals
10.3.2. Ambulatory Surgical Centers
10.3.3. Clinics & Orthopedic Centers
10.4. Market Attractiveness Analysis, by End-user
11. Global Interbody Fusion Cages Market Analysis and Forecast, by Region
11.1. Key Findings
11.2. Market Value Forecast, by Region, 2017–2031
11.2.1. North America
11.2.2. Europe
11.2.3. Asia Pacific
11.2.4. Latin America
11.2.5. Middle East & Africa
11.3. Market Attractiveness Analysis, by Region
12. North America Interbody Fusion Cages Market Analysis and Forecast
12.1. Introduction
12.1.1. Key Findings
12.2. Market Value Forecast, by Type, 2017–2031
12.2.1. Cervical
12.2.2. Lumbar
12.2.3. Others
12.3. Market Value Forecast, by Material , 2017–2031
12.3.1. Titanium
12.3.2. Polyetheretherketone (PEEK)
12.3.3. Others
12.4. Market Value Forecast, by Product, 2017–2031
12.4.1. Expandable Cages
12.4.2. Static Cages
12.5. Market Value Forecast, by Indication, 2017–2031
12.5.1. Degenerative Disc Disease
12.5.2. Spondylolisthesis
12.5.3. Spinal Tumors or Masse
12.5.4. Spinal Stenosis
12.5.5. Others
12.6. Market Value Forecast, by End-user, 2017–2031
12.6.1. Hospitals
12.6.2. Ambulatory Surgical Centers
12.6.3. Clinics & Orthopedic Centers
12.7. Market Value Forecast, by Country, 2017–2031
12.7.1. U.S.
12.7.2. Canada
12.8. Market Attractiveness Analysis
12.8.1. By Type
12.8.2. By Material
12.8.3. By Product
12.8.4. By Indication
12.8.5. By End-user
12.8.6. By Country
13. Europe Interbody Fusion Cages Market Analysis and Forecast
13.1. Introduction
13.1.1. Key Findings
13.2. Market Value Forecast, by Type, 2017–2031
13.2.1. Cervical
13.2.2. Lumbar
13.2.3. Others
13.3. Market Value Forecast, by Material , 2017–2031
13.3.1. Titanium
13.3.2. Polyetheretherketone (PEEK)
13.3.3. Others
13.4. Market Value Forecast, by Product, 2017–2031
13.4.1. Expandable Cages
13.4.2. Static Cages
13.5. Market Value Forecast, by Indication, 2017–2031
13.5.1. Degenerative Disc Disease
13.5.2. Spondylolisthesis
13.5.3. Spinal Tumors or Masse
13.5.4. Spinal Stenosis
13.5.5. Others
13.6. Market Value Forecast, by End-user, 2017–2031
13.6.1. Hospitals
13.6.2. Ambulatory Surgical Centers
13.6.3. Clinics & Orthopedic Centers
13.7. Market Value Forecast, by Country/Sub-region, 2017–2031
13.7.1. Germany
13.7.2. U.K.
13.7.3. France
13.7.4. Italy
13.7.5. Spain
13.7.6. Rest of Europe
13.8. Market Attractiveness Analysis
13.8.1. By Type
13.8.2. By Material
13.8.3. By Product
13.8.4. By Indication
13.8.5. By End-user
13.8.6. By Country/Sub-region
14. Asia Pacific Interbody Fusion Cages Market Analysis and Forecast
14.1. Introduction
14.1.1. Key Findings
14.2. Market Value Forecast, by Type, 2017–2031
14.2.1. Cervical
14.2.2. Lumbar
14.2.3. Others
14.3. Market Value Forecast, by Material , 2017–2031
14.3.1. Titanium
14.3.2. Polyetheretherketone (PEEK)
14.3.3. Others
14.4. Market Value Forecast, by Product, 2017–2031
14.4.1. Expandable Cages
14.4.2. Static Cages
14.5. Market Value Forecast, by Indication, 2017–2031
14.5.1. Degenerative Disc Disease
14.5.2. Spondylolisthesis
14.5.3. Spinal Tumors or Masse
14.5.4. Spinal Stenosis
14.5.5. Others
14.6. Market Value Forecast, by End-user, 2017–2031
14.6.1. Hospitals
14.6.2. Ambulatory Surgical Centers
14.6.3. Clinics & Orthopedic Centers
14.7. Market Value Forecast, by Country/Sub-region, 2017–2031
14.7.1. China
14.7.2. Japan
14.7.3. India
14.7.4. Australia & New Zealand
14.7.5. Rest of Asia Pacific
14.8. Market Attractiveness Analysis
14.8.1. By Type
14.8.2. By Material
14.8.3. By Product
14.8.4. By Indication
14.8.5. By End-user
14.8.6. By Country/Sub-region
15. Latin America Interbody Fusion Cages Market Analysis and Forecast
15.1. Introduction
15.1.1. Key Findings
15.2. Market Value Forecast, by Type, 2017–2031
15.2.1. Cervical
15.2.2. Lumbar
15.2.3. Others
15.3. Market Value Forecast, by Material , 2017–2031
15.3.1. Titanium
15.3.2. Polyetheretherketone (PEEK)
15.3.3. Others
15.4. Market Value Forecast, by Product, 2017–2031
15.4.1. Expandable Cages
15.4.2. Static Cages
15.5. Market Value Forecast, by Indication, 2017–2031
15.5.1. Degenerative Disc Disease
15.5.2. Spondylolisthesis
15.5.3. Spinal Tumors or Masse
15.5.4. Spinal Stenosis
15.5.5. Others
15.6. Market Value Forecast, by End-user, 2017–2031
15.6.1. Hospitals
15.6.2. Ambulatory Surgical Centers
15.6.3. Clinics & Orthopedic Centers
15.7. Market Value Forecast, by Country/Sub-region, 2017–2031
15.7.1. Brazil
15.7.2. Mexico
15.7.3. Rest of Latin America
15.8. Market Attractiveness Analysis
15.8.1. By Type
15.8.2. By Material
15.8.3. By Product
15.8.4. By Indication
15.8.5. By End-user
15.8.6. By Country/Sub-region
16. Middle East & Africa Interbody Fusion Cages Market Analysis and Forecast
16.1. Introduction
16.1.1. Key Findings
16.2. Market Value Forecast, by Type, 2017–2031
16.2.1. Cervical
16.2.2. Lumbar
16.2.3. Others
16.3. Market Value Forecast, by Material , 2017–2031
16.3.1. Titanium
16.3.2. Polyetheretherketone (PEEK)
16.3.3. Others
16.4. Market Value Forecast, by Product, 2017–2031
16.4.1. Expandable Cages
16.4.2. Static Cages
16.5. Market Value Forecast, by Indication, 2017–2031
16.5.1. Degenerative Disc Disease
16.5.2. Spondylolisthesis
16.5.3. Spinal Tumors or Masse
16.5.4. Spinal Stenosis
16.5.5. Others
16.6. Market Value Forecast, by End-user, 2017–2031
16.6.1. Hospitals
16.6.2. Ambulatory Surgical Centers
16.6.3. Clinics & Orthopedic Centers
16.7. Market Value Forecast, by Country/Sub-region, 2017–2031
16.7.1. GCC Countries
16.7.2. South Africa
16.7.3. Rest of Middle East & Africa
16.8. Market Attractiveness Analysis
16.8.1. By Type
16.8.2. By Material
16.8.3. By Product
16.8.4. By Indication
16.8.5. By End-user
16.8.6. By Country/Sub-region
17. Competition Landscape
17.1. Market Player – Competition Matrix (by tier and size of companies)
17.2. Market Share Analysis, by Company (2022)
17.3. Company Profiles
17.3.1. Medtronic plc
17.3.1.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.1.2. Product Portfolio
17.3.1.3. Financial Overview
17.3.1.4. SWOT Analysis
17.3.1.5. Strategic Overview
17.3.2. Johnson & Johnson
17.3.2.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.2.2. Product Portfolio
17.3.2.3. Financial Overview
17.3.2.4. SWOT Analysis
17.3.2.5. Strategic Overview
17.3.3. Stryker Corporation
17.3.3.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.3.2. Product Portfolio
17.3.3.3. Financial Overview
17.3.3.4. SWOT Analysis
17.3.3.5. Strategic Overview
17.3.4. Zimmer Biomet
17.3.4.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.4.2. Product Portfolio
17.3.4.3. Financial Overview
17.3.4.4. SWOT Analysis
17.3.4.5. Strategic Overview
17.3.5. B. Braun Melsungen AG
17.3.5.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.5.2. Product Portfolio
17.3.5.3. Financial Overview
17.3.5.4. SWOT Analysis
17.3.5.5. Strategic Overview
17.3.6. NuVasive, Inc.
17.3.6.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.6.2. Product Portfolio
17.3.6.3. Financial Overview
17.3.6.4. SWOT Analysis
17.3.6.5. Strategic Overview
17.3.7. Alphatec Holdings, Inc.
17.3.7.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.7.2. Product Portfolio
17.3.7.3. Financial Overview
17.3.7.4. SWOT Analysis
17.3.7.5. Strategic Overview
17.3.8. LASAK s.r.o.
17.3.8.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.8.2. Product Portfolio
17.3.8.3. Financial Overview
17.3.8.4. SWOT Analysis
17.3.8.5. Strategic Overview
17.3.9. Xtant Medical Holdings, Inc.
17.3.9.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.9.2. Product Portfolio
17.3.9.3. Financial Overview
17.3.9.4. SWOT Analysis
17.3.9.5. Strategic Overview
17.3.10. Spineology, Inc.
17.3.10.1. Company Overview (HQ, Business Segments, Employee Strength)
17.3.10.2. Product Portfolio
17.3.10.3. Financial Overview
17.3.10.4. SWOT Analysis
17.3.10.5. Strategic Overview
List of Tables
Table 01: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 02: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 03: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 04: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 05: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 06: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Region, 2017–2031
Table 07: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 08: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 09: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 10: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 11: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 12: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 13: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 14: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 15: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 16: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 17: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 18: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 19: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 20: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 21: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 22: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 23: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 24: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 25: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 26: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 27: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 28: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 29: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
Table 30: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Country/Sub-region, 2017–2031
Table 31: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Type, 2017–2031
Table 32: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Material, 2017–2031
Table 33: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Product, 2017–2031
Table 34: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by Indication, 2017–2031
Table 35: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast, by End-user, 2017–2031
List of Figures
Figure 01: Global Interbody Fusion Cages Market Value (US$ Mn) Forecast, 2017–2031
Figure 02: Global Interbody Fusion Cages Market Value Share, by Type, 2022
Figure 03: Global Interbody Fusion Cages Market Value Share, by Material, 2022
Figure 04: Global Interbody Fusion Cages Market Value Share, by Product, 2022
Figure 05: Global Interbody Fusion Cages Market Value Share, by Indication, 2022
Figure 06: Global Interbody Fusion Cages Market Value Share, by End-user, 2022
Figure 07: Global Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 08: Global Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 09: Global Interbody Fusion Cages Market Value Share Analysis, by Material 2022 and 2031
Figure 10: Global Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 11: Global Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 12: Global Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 13: Global Interbody Fusion Cages Market Value Share Analysis, by Indication 2022 and 2031
Figure 14: Global Interbody Fusion Cages Market Attractiveness Analysis, Indication, 2023–2031
Figure 15: Global Interbody Fusion Cages Market Value Share Analysis, by End-user 2022 and 2031
Figure 16: Global Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 17: Global Interbody Fusion Cages Market Value Share Analysis, by Country, 2022 and 2031
Figure 18: Global Interbody Fusion Cages Market Attractiveness Analysis, by Country, 2023–2031
Figure 19: North America Interbody Fusion Cages Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 20: North America Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 21: North America Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 22: North America Interbody Fusion Cages Market Value Share Analysis, by Material 2022 and 2031
Figure 23: North America Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 24: North America Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 25: North America Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 26: North America Interbody Fusion Cages Market Value Share Analysis, by Indication 2022 and 2031
Figure 27: North America Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 28: North America Interbody Fusion Cages Market Value Share Analysis, by End-user 2022 and 2031
Figure 29: Europe Interbody Fusion Cages Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 30: Europe Interbody Fusion Cages Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 31: Europe Interbody Fusion Cages Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 32: Europe Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 33: Europe Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 34: Europe Interbody Fusion Cages Market Value Share Analysis, by Material, 2022 and 2031
Figure 35: Europe Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 36: Europe Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 37: Europe Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 38: Europe Interbody Fusion Cages Market Value Share Analysis, by Indication, 2022 and 2031
Figure 39: Europe Interbody Fusion Cages Market Attractiveness Analysis, Indication, 2023–2031
Figure 40: Europe Interbody Fusion Cages Market Value Share Analysis, by End-user, 2022 and 2031
Figure 41: Europe Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 42: Asia Pacific Interbody Fusion Cages Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 43: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 44: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 45: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 46: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 47: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by Material, 2022 and 2031
Figure 48: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 49: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 50: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 51: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by Indication, 2022 and 2031
Figure 52: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, Indication, 2023–2031
Figure 53: Asia Pacific Interbody Fusion Cages Market Value Share Analysis, by End-user, 2022 and 2031
Figure 54: Asia Pacific Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 55: Latin America Interbody Fusion Cages Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 56: Latin America Interbody Fusion Cages Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 57: Latin America Interbody Fusion Cages Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 58: Latin America Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 59: Latin America Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 60: Latin America Interbody Fusion Cages Market Value Share Analysis, by Material, 2022 and 2031
Figure 61: Latin America Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 62: Latin America Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 63: Latin America Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 64: Latin America Interbody Fusion Cages Market Value Share Analysis, by Indication, 2022 and 2031
Figure 65: Latin America Interbody Fusion Cages Market Attractiveness Analysis, Indication, 2023–2031
Figure 66: Latin America Interbody Fusion Cages Market Value Share Analysis, by End-user, 2022 and 2031
Figure 67: Latin America Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 68: Middle East & Africa Interbody Fusion Cages Market Value (US$ Mn) Forecast and Y-o-Y Growth (%) Projection, 2017–2031
Figure 69: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by Country/Sub-region, 2022 and 2031
Figure 70: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, by Country/Sub-region, 2023–2031
Figure 71: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by Type 2022 and 2031
Figure 72: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, Type, 2023–2031
Figure 73: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by Material, 2022 and 2031
Figure 74: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, Material, 2023–2031
Figure 75: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by Product 2022 and 2031
Figure 76: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, Product, 2023–2031
Figure 77: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by Indication, 2022 and 2031
Figure 78: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, Indication, 2023–2031
Figure 79: Middle East & Africa Interbody Fusion Cages Market Value Share Analysis, by End-user, 2022 and 2031
Figure 80: Middle East & Africa Interbody Fusion Cages Market Attractiveness Analysis, End-user, 2023–2031
Figure 81: Global Interbody Fusion Cages Market Share Analysis, by Company, 2022